Todd:Pictet-Spengler to PZQ: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
No edit summary |
No edit summary |
||
| Line 26: | Line 26: | ||
===Synthesis of the catalysts=== | ===Synthesis of the catalysts=== | ||
'''N,N’-bis-3,5-bis(trifluoromethyl)phenyl-thiourea''' | |||
[[Image:N,N’-bis-3,5-bis(trifluoromethyl)phenyl-thiourea.png]] | |||
* Acid-free, organocatalytic acetalization, M. Kotke and P. R. Schreiner, Tetrahedron 2006, 62, 2-3, 434-439; [http://dx.doi.org/10.1016/j.tet.2005.09.079 doi:10.1016/j.tet.2005.09.079]. | |||
* Synthetic Studies toward Aryl-(4-aryl-4H-[1,2,4]triazole-3-yl)-amine from 1,3-Diarylthiourea as Urea Mimetics, A. Natarajan, Y. Guo, H. Arthanari, G. Wagner, J. A. Halperin and M. Chorev, J. Org. Chem. 2005, 70, 16, 6362–6368; [http://dx.doi.org/10.1021/jo0508189 DOI: 10.1021/jo0508189]. | |||
'''1,1-Binaphthyl-2,2-disulfonate''' | '''1,1-Binaphthyl-2,2-disulfonate''' | ||
Revision as of 23:52, 24 May 2011
Pictet-Spengler route to Praziquantel
Michael Woelfle,1 and Matthew H. Todd1*
1. School of Chemistry, The University of Sydney, NSW 2006, Australia
- To whom correspondence should be addressed: Tel. +61 2 9351 2180, matthew.todd@sydney.edu.au
Abstract
The Pictet-Spengler route to Praziquantel
The PZQ precursor
By using the Ugi reaction
Introduction
Results
Preparation of the Ugi-intermediates
Synthesis of the catalysts
N,N’-bis-3,5-bis(trifluoromethyl)phenyl-thiourea
- Acid-free, organocatalytic acetalization, M. Kotke and P. R. Schreiner, Tetrahedron 2006, 62, 2-3, 434-439; doi:10.1016/j.tet.2005.09.079.
- Synthetic Studies toward Aryl-(4-aryl-4H-[1,2,4]triazole-3-yl)-amine from 1,3-Diarylthiourea as Urea Mimetics, A. Natarajan, Y. Guo, H. Arthanari, G. Wagner, J. A. Halperin and M. Chorev, J. Org. Chem. 2005, 70, 16, 6362–6368; DOI: 10.1021/jo0508189.
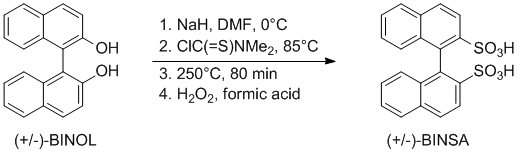
1,1-Binaphthyl-2,2-disulfonate
1. Step: Preparation of 1,1’-Binaphthalene-2,2’-diyl-O,O’-bis(N,N’-dimethylthiocarbamate) (MW45-1)
2. Step: Preparation of 1,1’-Binaphthalene-2,2’-diyl-S,S’-bis(N,N’-dimethylthiocarbamate) (MW45-2)
3. Step: Preparation of 1,1’-Binaphthalene-2,2’-disulfonic acid (MW45-3)
- Pyridinium 1,1′-Binaphthyl-2,2′-disulfonates as Highly Effective Chiral Brønsted Acid−Base Combined Salt Catalysts for Enantioselective Mannich-Type Reaction, M. Hatano, T. Maki, K. Moriyama, M. Arinobe and K. Ishihara, J. Am. Chem. Soc. 2008, 130, 16858–16860; DOI: 10.1021/ja806875c.
- A Powerful Chiral Counteranion Motif for Asymmetric Catalysis, P. García-García, F. Lay, P. García-García, C. Rabalakos, B. List, Angew. Chem. Int. Ed. 2009, 48, 4363 –4366; DOI: 10.1002/anie.200901768.
References
- Synthesis of Praziquantel, J. H. Kim, Y. S. Lee, H. Park and C. S. Rim, Tetrahedron , 1998, 54, 7395-7400. (DOI: doi:10.1016/S0040-4020(98)00401-3)