Treating Spinal Cord Injuries With Stem Cell Therapy, by Nicole Raia: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Template:ChemEng590B}} | |||
==Background on Stem Cells== | ==Background on Stem Cells== | ||
Latest revision as of 12:34, 18 January 2013
Background on Stem Cells
Definition
Stem cells are unique in the fact that they have three specific characteristics that are unlike those of most cells. First, they are capable of proliferating as stem cells for extended periods of time. Second, they are undifferentiated. And third, they can give rise to other cell types. All of these characteristics show the promise for stem cells used for medical purposes. [1]
Embryonic Stem Cells
Embryonic stem cells (ESCs) are specific stem cells that come from an embryo. These pluripotent cells can be grown in the laboratory for years without differentiated. Nanog and Oct4 are specific transcription factors that prove the cells in culture are ESCs. [1] ESCs can give rise to specific specialty cells such as, adipocytes, neurons, macrophages, smooth muscle, astrocytes, and oligodendrocytes given the correct environmental conditions [2]. This differentiation does not occur with a single step but more realistically occurs over multiple steps involving progenitor and precursor cells. [1]
Stem Cell Therapy Promise
Stem cell properties allow them to treat a variety of diseases. Diseases that can possibly be treated with stem cell therapy can range from type I diabetes, to neurodegenerative diseases, to soft tissue injuries. This cell-based therapy is also known as regenerative or reparative medicine. This promise of stem cell based therapies induced an increase in research to find new ways to not only treat but also possibly heal specific diseases and/or injuries. [1]
Stem Cell History
The mid-1800’s can be considered the start of stem cell research. This marked the discovery that cells can actually general other cells. It was not until 1998 that the first human embryonic stem cell (hESC) was isolated and grown in a laboratory setting by James Thompson at the University of Wisconsin-Madison [3]. Since then hESCs have been a controversial research topic in politics. In 2009, President Obama finally lifts the ban that was placed upon hESC research in 2001 by President Bush. Hence in 2010, Geron Corporation initiated the first clinical trials of hESC-based therapy to heal acute spinal injury [4].

The Spinal Cord
Definition
The spinal cord runs along the vertebrae and is where all of the the major nerves in the body meet. It helps facilitate the control of not only motor functions of the extremities but also daily functions of organs in the body. Injury to this area can be detrimental. [6]
Spinal Cord Injuries
Spinal cord injuries (SCIs) can be caused from numerous things such as, trauma, loss of normal blood supply, or compression. The most common cause of SCIs is trauma. This is often due to falling or direct wounds such as gunshot wounds or stabbings. SCIs can be complete or incomplete. A complete SCI is characterized by the loss of function below the level of injury where an incomplete SCI patient still maintains some or all function. Complete SCIs can lead to quadriplegia (paralysis below injury including the arms and legs) or paraplegia (paralysis below injury including only the legs). [6] There are around 12,000 new cases of SCIs each year in the United States. It was also found to cost an extra $1 million to $4.3 million for health care and living expenses for a single patient with an SCI. [7]
Past and Present SCI Treatments
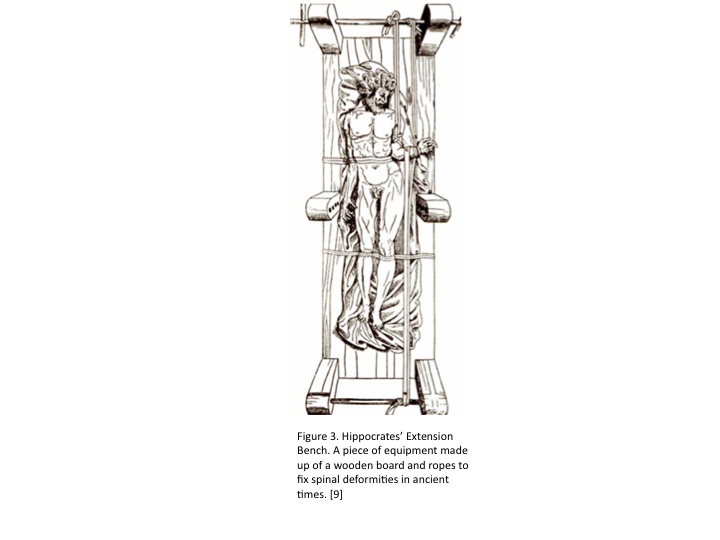
In ancient times, SCIs were treated by rest, immobilization, and traction. In Greece, Hippocrates invented an extension bench, which provides a person suffering from an SCI or a spine deformity with immobilization and traction using a stiff board and ropes. It was not until after WWII, did the treatment of spinal cord injuries evolved, yet they still remained fairly poor and inefficient. [8]
Today, there is only treatment for SCIs. SCIs are most commonly treated with surgery, immobilization, traction, and rehabilitation. Much current treatment focuses on rehabilitation, which provides SCI patients with new way of living to accommodate for their injury. Currently, there is no cure for SCIs. [6]
hESCs can Treat SCIs
Since the early 1900s, cell based therapies have been researched to find a better treatment or possibly even a cure for SCIs. Since hESCs are pluripotent, they can give rise to cells that are involved in the central nervous system. Given the right conditions, an hESC can differentiated into an oligodendrocyte progenitor cell (OPC). With the correct environment and given the correct growth factors OPCs can form a fully mature oligodendrocyte cell. Some growth factors that are known to be involved in this process include fibroblast, epidermal, and platelet-derived growth factors. Also the use of retinoic acid in cultures have been shown to give rise the neuronal cells. [10]
Oligodendrocytes are classified as glial cells. Glial cells are directly associated with the central nervous system. Oligodendrocytes help develop neurons correctly and are specifically involved with myelinating axons of cells. The myelin sheath along the axon of a cell helps propagate action potential down the cell, allowing signals to reach other cells. This signaling process is essential to the central nervous system and results in functioning in the body (eg. Muscle movement). [11] When an SCI occurs, the cells in the spinal cord can become damaged. This causes the transmittance of signals to be disrupted resulting in SCI symptoms such as paralysis. Oligodendrocytes can help fix these damaged cells by repairing axons via re-myelination. This can increase the success of action potential traveling, promoting signal propagation, and potentially restore motor function. So many believe that by using hESC-based therapy, SCIs can be cured. [10]
Experiments using hESCs to Treat SCIs
ESCs Differentiation into Oligodendrocytes
Su Liu (Washington University School of Medicine and the Center for the Study of Nervous System Injury) published one of the first papers that showed the ides of ESCs differentiating into oligodendrocytes to help treat SCIs. The research proposed in the paper showed the promise of finding a cure to these currently non-curable injuries. In this experiment, ESCs were differentiated into oligodendrocytes. These ESC-derived cells were then shown to myelinate axons in vitro. Through this experiment, they also showed that when ESCs were inserted into a rat’s central nervous system, the cells differentiated into mature oligodendrocytes which are known be cause myelination (via in vitro studies).
Hans S. Keirstead and Geron Corporation
In 2005, Hans S. Keirstead published his studies on how hESC-derived oligodendrocyte progenitor cells can be transplanted and cure rats with SCIs. Keirstead started by inducing differentiation of hESCs in order to create high purity OPCs. These OPCs were then transplanted into the spinal cords of rats with SCIs. Transplanting OPCs instead of pure undifferentiated hESCs reduced the risk of hESCs differentiated into other cell types and also reduced the formation of teratomas. It was found that after the OPCs were transplanted to the spinal cord, they formed into fully mature oligodendrocytes and helped to myelinate damaged axons. This result was only found when OPC treatment occurred 7 days after the injury was implemented. The treatment failed when given 10 months after the occurrence of the injury. The success of this experiment showed how hESC-based therapy soon after SCI could help remyelinate spinal cord cell axons and restore an organism’s locomotive function. [12]
In 2010, Geron Corporation started clinical trials using hESC-based therapy to treat SCIs based on Keirstead’s work. In 2011, Geron stopped recruiting subjects due to financial reasons and their need to focus on cancer research but continue collecting data using the patients already enrolled. This study is titled: Phase 1 Safety Study of GRNOPC1 in Patients With Neurologically Complete, Subacute, Spinal Cord Injury. GRNOPC1 is the population of cells that have hESC-derived OPCs. In order for human trials to be conducted, the safety of inserting GRNOPC1 was determined via animal studies. This cell transplantation resulted in no teratomas 1 year after transplantation, small, non-harmful cysts at injection sites, no toxicity or cell migration outside the central nervous system, no stimulated pain, and no major susceptibility to immune attack. [13]

Results and Side Effects
In October 2011, the data from Phase I was released. Geron found that there were no surgical complications, no serious adverse events, mild adverse events due to immunosuppressive drugs (nausea and low magnesium levels), no neurological changes, no injury to spinal cord due to injection, and no evidence of immune responses. It is estimated that Geron will complete final primary data collection in October 2012. [13]
Other Possible Treatments for SCIs using Non-cell Based Techniques
Baylor College of Medicine and Integra LifeSciences supported an experiment to treat SCIs involving decorin, a small proteoglycan that is primarily found in extracellular matrix. It has been found that condroitin sulfate proteoglycans (CSPGs) are expressed in excess when SCIs occur. These proteins inhibit axon growth, leading to such things as paralysis. CSPGs are induced by transform growth factor beta (TGFß) and epidermal growth factor receptor (EGFR). Decorin has the ability to limit TGFß and EGFR, decreasing the expression of CSPGs, and increasing axon reformation and spinal cord function. [14]
In 2004, Jeannette E. Davies published an experiment to show how decorin can support axon growth. Decorin was inserted to rat spinal cords via cannulas. This resulted in a decreased immunoreactivity for CSPGs. In vitro, a co-culture of fibroblast and neuronal cells from rat spinal columns were tested with decorin. This resulted in axon growth. This shows the promise of protein-derived therapy can help treat and possibly cure SCIs. [14]
References
[1] Stem Cells: Scientific Progress and Future Research Directions. Department of Health and Human Services. June 2001. </info/scireport/2001report>.
[2] Alberts, Bruce. Molecular Biology of the Cell. New York: Garland Science, 2008.
[3] History of Stem Cell Research. <http://www.allaboutpopularissues.org/history-of-stem-cell-research-faq.htm>
[4]Stem Cell History <http://stemcellhistory.com/>
[5]Strassman, Marc. Korean team led by Hwang Woo-suk creates 11 lines of human embryonic stem cells from afflicted patients; ethical issues remain. 2005. <http://www.etopiamedia.net/emmnn/pages/emmnn70-5551212.html>
[6]Eck, C.J. and J.W. Marks. Spinal Cord Injury. <http://www.medicinenet.com/spinal_cord_injury_treatments_and_rehabilitation/article.htm>
[7]Spinal Cord Injury Facts and Figures at a Glance. National Spinal Cord Injury Statistical Center, Birminghanm, Alabama. 2011. <http://www.synapsebiomedical.com/support/PDFs/SCI_Reimbursement/SCI%20Facst%20and%20Figures%20NSCISC%202011%20Update.pdf>
[8] Eltorai, I.M. History of Spinal Cord Medicine. <http://www.demosmedpub.com/files/LinChapterOne.pdf>
[9]Vasiliadis, E.S, T.B Grivas, and A. Kaspiris. Historical overview of spinal deformities in ancient Greece. Scoliosis (4):6. 2009.<http://www.scoliosisjournal.com/content/4/1/6>
[10] Liu S., et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. PNAS (97): 6126-31. 2000.
[11] Baumann, N. and D. Pham-Dinh. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological Reviews (81): 871-927. 2001
[12] Keirstead, H.S., et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. The Journal of Neuroscience (19): 4694-4705. 2005
[13] Geron Corporation. Programs. 2012. http://cell-therapies.geron.com
[14] Davies, J.E. et al. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. European Journal of Neuroscience (19):1226-42. 2004