User:Ajayi Pickering-Haynes/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| Line 27: | Line 27: | ||
Conclusion: Upon observing under the microscope and use of the dichotomous keys, we included that our transect is indeed a rather diverse environment containing Algae and Protists based on our Hayculture sample. After taking samples from various niches (locations) of our Hayculture we concluded that it contained Chlamydomonas and Peranema. The morphological characteristics of these organisms allowed us to use the keys provided to come to these conclusions. | Conclusion: Upon observing under the microscope and use of the dichotomous keys, we included that our transect is indeed a rather diverse environment containing Algae and Protists based on our Hayculture sample. After taking samples from various niches (locations) of our Hayculture we concluded that it contained Chlamydomonas and Peranema. The morphological characteristics of these organisms allowed us to use the keys provided to come to these conclusions. | ||
Lab #3 - Microbiology & Identifying Bacteria | |||
Hay Culture Observation: Much of the Hay Culture stayed the same. However the smell became stronger and more acrid, which could account for the growth of more micro-organisms. As temperature has an ideal effect on growth, the lab environment might have caused these slight changes to our Hay Culture. The agar plates are used to test the presence of bacteria and thus Archaea would probably be unlikely to grow on the plates because they are metabolically different, and thus the presence of Archaea might have to be tested differently. | |||
Introduction: Bacteria are diverse in shape, metabolism, and function. Through morphological observation bacteria can be identified. The three basic shapes are bacillus which are rod-shaped, coccus which are spherical, and spirillum which are twisted spiral (Bio 210 Laboratory Manual). Another morphological characteristic commonly used by scientists is the stain of bacteria which reflect the presence of peptidoglycan in the cell wall of bacteria. Bacteria are considered to be gram-positive, when they have a thicker presence of peptidoglycan with a blue stain and gram-negative when less peptidoglycan is present with a pink stain apparent on the microscope. In addition to these morphological observations, through the use of a polymerase chain reaction bacteria's metabolic activity can be determined by observing the difference that lie in DNA sequence among the genes of different bacteria. In this lab procedure, it is predicted that a myriad of diverse bacteria will be observed from our Hay Infusion Culture which reflect that transect 2 contains a diverse collection of abundant life. | |||
Methods & Materials: First, transect 2 Hay Culture was observed for any changes in appearance, smell, or distinct features. Thereafter, the agar plates made the week before were observed. An estimation of colonies on each plate were recorded and the elevation and shape were determined. In addition other features of the bacteria plated were observed to determine how much species each plate contained. After making these conclusions, two micro-organisms from the nutrient agar and two from the nutrient agar with tetracycline plates were used to make wet mounts and gram stains. Both were observed under a microscope to characterize bacteria and observe the size. The wet mount confirmed the presence of bacteria observing its motility and the gram stain confirmed the bacteria as gram positive or negative based on the pigment. To conclude the lab experiment, two of the classified bacteria's 16S rRNA genes were amplified by use of primers and PCR to be observed the following next week. | |||
Results: | |||
There are some differences apparent in the number of colonies between plates that contain antibiotics and those that don't. However, only 2 species of bacteria are apparent in the plates upon observation of the pigment. A white and orange pigment was observed in plates that only contained a few colonies. In dilution agar plates with tetracycline 10^-3, and 10^-9 more colonies of bacteria were counted present than the nutrient agar plate without the antibiotic. Since only 2 species were apparent within the plates, only two were unaffected by the tetracycline. Amongst all the plates, the nutrient agar plates collectively contain 241 colonies, while the nutrient with tetracycline plates contain 349 collectively. The Tetracycline 10^-9 wet mount showed that the bacteria were motile, small transparent clear dots and about 20 colonies were visible on the microscope. The nutrient 10^-9 wet mount appeared motile as well, contained one large colony moving together and about 50 colonies were visible underneath the microscope. The Tetracycline 10^-5 wet mount appeared with a green-purplish pigment and about 60 colonies were seen under the microscope. To conclude, wet mount observations the 10^-5 appeared to have a circular shape and about a few colonies present, however this wet mount showed no motile and was believed to had dried out by this observation. | |||
For gram stain observation, 10^-9 nutrient agar plate appeared to be rough and have a deep purple pigment, it was lobate and had slight irregularities and was concluded as gram positive. | |||
The 10^-5 tetracycline plate was pinkish red with an elongated shape, circular, and concluded to be gram negative. The 10^-5 nutrient plate was a bluish purple with an irregularly circular shape, and was concluded to be gram positive. To conclude gram stain observations, the 10^-9 tetracycline plate contained a dark blue pigment with an irregular shape and was concluded as gram positive. | |||
Nutrient Agar & Tetracycline + Nutrient Agar Plate Pictures: | |||
[[Image:IMG_1498.jpg]] | |||
Serial Dilutions Table: | |||
[[Image:SerialDilutionsTable.jpg]] | |||
Nutrient Agar 10^-9 Wet Mount | |||
[[Image:wetmountpic1.jpg]] | |||
Tetracycline 10^-9 Wet Mount | |||
[[Image:wetmountpic2.jpg]] | |||
10^-9 Nutrient Agar Plate Gram Stain | |||
[[Image:IMG_1513.jpg]] | |||
10^-9 Tetracycline Nutrient Plate Gram Stain | |||
[[Image:IMG_1521.jpg]] | |||
10^-5 Tetracycline Plate Gram Stain | |||
[[Image:IMG_1526.jpg]] | |||
10^-5 Nutrient Agar Gram Stain | |||
[[Image:IMG_1532.jpg]] | |||
Discussion: Using the serial dilution plates to observe the colony morphology reflects the idea that antibiotic resistance is high on the rise. 10^-3 and 10^-9 tetracycline plates contained more bacterial colonies, which means they are resistant to the antibiotic. Overall, there were bacterial colonies amongst all the tetracycline plates than there were in the ones within the antibiotic. This indicates that this antibiotic is indeed feeding the bacteria and that it has no profound effect on the bacterial growth. Tetracycline would commonly kill bacteria or inhibit the growth of bacterial colonies that it has a mechanism of action on, hence why it is used as a variable (Bio 210 Lab Manual). Its hard to tell which kind of bacteria based on gram stain this specific antibiotic has an effect on. As in the 10^-9 gram positive plates more bacteria were found on the tetracycline plate, whereas in the 10^-5 gram positive for nutrient and gram negative for the tetracycline containing, the one with the tetracycline containing had a few more colonies of bacteria. One can suggest that from our experiment maybe tetracycline has a more significant effect on gram negative bacteria, however further experimentation would need to be carried out to confirm this. Amongst the gram stain procedures, three bacterial colony groups were positive and one was negative. This gram negative bacteria contained the only pinkish red pigment and was affected by tetracycline in terms of growth. From this, we might be able to hypothetically conclude that tetracycline has an effect on a certain bacteria causing the peptidoglycan presence within the cell wall to lessen, thus why the bacteria is gram-negative. | |||
Revision as of 16:23, 4 February 2016
Transect #2 Lab 1 : Transect Observation and Hayculture
Transect #2 was in the Amphitheater area of the American University campus. It was located in the front of a building containing a stream in the central of its location. It was surrounded by several trees, shrubs, plant life, soil, rocks, and dirt. The stream was constantly flowing with rocks, soil, and a green pigment which suggested a presence of algae or protists. The biotic elements of our location are all the natural life components like trees, microorganisms, and other plant organisms. The area also contained an abundance of abiotic factors that stem from sunlight to the soil, and the temperature which was rather cold in despite of the sun being out. The transect was considerably rocky containing a pathway of rocks from its opening to the stream that existed in the middle. On the outskirts of the transect were colonies of different plant life, bare trees, and the ground was filled with leaves and branches. In essence the topography of the transect was a square solid structure where the stream lay ahead from west to east, the pathway of rocks went from south to the north, and the building behind our transect marked the northern location. 




Lab 2 : Protist & Algae Identification
Description: Our Hay Culture Infusion was made from Transect 2 which was by the Amphitheater of the American University. It was made from a collection of dirt, soil, and the environment from our area. The Culture was made from our sample outside, with 500 milliliters of water, 0.1 grams of dried milk, and laid out to settle for approximately a week before observation. Upon observation of the culture we observed a rather dark pigment filled with mold and a large amount of protists and algae. This confirmed that life was apparent within the sample. Our culture contained a strong acrid smell, and appeared to be harmful and pathogenic to the eye.
Materials & Methods: Our first niche was taken from the bottom of the Hayculture which contained mostly dirt and a few moving organisms apparent. After taking a went mount sample of that niche, we used a microscope to observe features that would allow us to classify the organisms and learn about its specialized characteristics. Niche #1 from the bottom of the hayculture proved to be a form of Algae, formally known as Chlamydomonas. Through use of our dichotomous key, after first observing that our sample was green, we followed the pattern and confirmed that it was a grass-green color, a free swimming algae with flagella, and that the cells were solitary and globular which led us to classify this Algae as Chlamydomonas. Next, we choose a sample from the middle of the culture which was labeled as niche #2. This sample also proved to be an Algae, formally known as Chlamydomonas as well. Containing the same size and appearance as sample 1 with a green pigment and solitary motile characteristics, we concluded this to be the same through the use of the key again. Lastly, we drew a sample from the top of our Hayculture which we labeled as niche #3. This sample turned out to be a Protist in contrast to the other two, formally known as Peranema. Following our key, we observed that it was colorless, exhibited a differing form of motion, contained no presence of cilia, was all single, motile cells in the sample, contained one observed locomotor flagella, and appeared very vibrantly when in motion. These characteristics led us to the conclusion that this Protist was Peranema specifically. To conclude these observations we made serial dilutions to plate organisms present in our cultures on nutrient agar and tetracyclin plates.
Organisms Identified & Serial Dilution Pictures:
Conclusion: Upon observing under the microscope and use of the dichotomous keys, we included that our transect is indeed a rather diverse environment containing Algae and Protists based on our Hayculture sample. After taking samples from various niches (locations) of our Hayculture we concluded that it contained Chlamydomonas and Peranema. The morphological characteristics of these organisms allowed us to use the keys provided to come to these conclusions.
Lab #3 - Microbiology & Identifying Bacteria
Hay Culture Observation: Much of the Hay Culture stayed the same. However the smell became stronger and more acrid, which could account for the growth of more micro-organisms. As temperature has an ideal effect on growth, the lab environment might have caused these slight changes to our Hay Culture. The agar plates are used to test the presence of bacteria and thus Archaea would probably be unlikely to grow on the plates because they are metabolically different, and thus the presence of Archaea might have to be tested differently.
Introduction: Bacteria are diverse in shape, metabolism, and function. Through morphological observation bacteria can be identified. The three basic shapes are bacillus which are rod-shaped, coccus which are spherical, and spirillum which are twisted spiral (Bio 210 Laboratory Manual). Another morphological characteristic commonly used by scientists is the stain of bacteria which reflect the presence of peptidoglycan in the cell wall of bacteria. Bacteria are considered to be gram-positive, when they have a thicker presence of peptidoglycan with a blue stain and gram-negative when less peptidoglycan is present with a pink stain apparent on the microscope. In addition to these morphological observations, through the use of a polymerase chain reaction bacteria's metabolic activity can be determined by observing the difference that lie in DNA sequence among the genes of different bacteria. In this lab procedure, it is predicted that a myriad of diverse bacteria will be observed from our Hay Infusion Culture which reflect that transect 2 contains a diverse collection of abundant life.
Methods & Materials: First, transect 2 Hay Culture was observed for any changes in appearance, smell, or distinct features. Thereafter, the agar plates made the week before were observed. An estimation of colonies on each plate were recorded and the elevation and shape were determined. In addition other features of the bacteria plated were observed to determine how much species each plate contained. After making these conclusions, two micro-organisms from the nutrient agar and two from the nutrient agar with tetracycline plates were used to make wet mounts and gram stains. Both were observed under a microscope to characterize bacteria and observe the size. The wet mount confirmed the presence of bacteria observing its motility and the gram stain confirmed the bacteria as gram positive or negative based on the pigment. To conclude the lab experiment, two of the classified bacteria's 16S rRNA genes were amplified by use of primers and PCR to be observed the following next week.
Results:
There are some differences apparent in the number of colonies between plates that contain antibiotics and those that don't. However, only 2 species of bacteria are apparent in the plates upon observation of the pigment. A white and orange pigment was observed in plates that only contained a few colonies. In dilution agar plates with tetracycline 10^-3, and 10^-9 more colonies of bacteria were counted present than the nutrient agar plate without the antibiotic. Since only 2 species were apparent within the plates, only two were unaffected by the tetracycline. Amongst all the plates, the nutrient agar plates collectively contain 241 colonies, while the nutrient with tetracycline plates contain 349 collectively. The Tetracycline 10^-9 wet mount showed that the bacteria were motile, small transparent clear dots and about 20 colonies were visible on the microscope. The nutrient 10^-9 wet mount appeared motile as well, contained one large colony moving together and about 50 colonies were visible underneath the microscope. The Tetracycline 10^-5 wet mount appeared with a green-purplish pigment and about 60 colonies were seen under the microscope. To conclude, wet mount observations the 10^-5 appeared to have a circular shape and about a few colonies present, however this wet mount showed no motile and was believed to had dried out by this observation.
For gram stain observation, 10^-9 nutrient agar plate appeared to be rough and have a deep purple pigment, it was lobate and had slight irregularities and was concluded as gram positive.
The 10^-5 tetracycline plate was pinkish red with an elongated shape, circular, and concluded to be gram negative. The 10^-5 nutrient plate was a bluish purple with an irregularly circular shape, and was concluded to be gram positive. To conclude gram stain observations, the 10^-9 tetracycline plate contained a dark blue pigment with an irregular shape and was concluded as gram positive.
Nutrient Agar & Tetracycline + Nutrient Agar Plate Pictures:
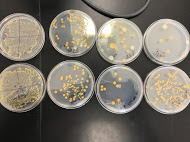
10^-9 Nutrient Agar Plate Gram Stain

10^-9 Tetracycline Nutrient Plate Gram Stain

10^-5 Tetracycline Plate Gram Stain

10^-5 Nutrient Agar Gram Stain

Discussion: Using the serial dilution plates to observe the colony morphology reflects the idea that antibiotic resistance is high on the rise. 10^-3 and 10^-9 tetracycline plates contained more bacterial colonies, which means they are resistant to the antibiotic. Overall, there were bacterial colonies amongst all the tetracycline plates than there were in the ones within the antibiotic. This indicates that this antibiotic is indeed feeding the bacteria and that it has no profound effect on the bacterial growth. Tetracycline would commonly kill bacteria or inhibit the growth of bacterial colonies that it has a mechanism of action on, hence why it is used as a variable (Bio 210 Lab Manual). Its hard to tell which kind of bacteria based on gram stain this specific antibiotic has an effect on. As in the 10^-9 gram positive plates more bacteria were found on the tetracycline plate, whereas in the 10^-5 gram positive for nutrient and gram negative for the tetracycline containing, the one with the tetracycline containing had a few more colonies of bacteria. One can suggest that from our experiment maybe tetracycline has a more significant effect on gram negative bacteria, however further experimentation would need to be carried out to confirm this. Amongst the gram stain procedures, three bacterial colony groups were positive and one was negative. This gram negative bacteria contained the only pinkish red pigment and was affected by tetracycline in terms of growth. From this, we might be able to hypothetically conclude that tetracycline has an effect on a certain bacteria causing the peptidoglycan presence within the cell wall to lessen, thus why the bacteria is gram-negative.









