User:Alexander Cvitan/Notebook/Experimental Biological Chemistry Lab/2013/08/28: Difference between revisions
From OpenWetWare
No edit summary |
No edit summary |
||
| Line 36: | Line 36: | ||
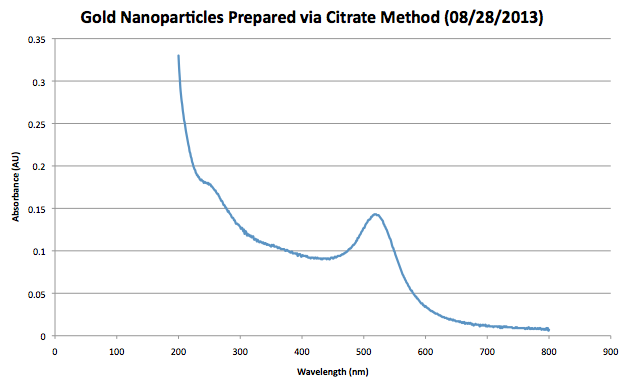
*Absorbance at 450 nm = .091 AU<br.> | *Absorbance at 450 nm = .091 AU<br.> | ||
*Using the reference above the particle diameter is 12 nm and the molar absorptivity is 1.09*10^8<br.> | *Using the reference above the particle diameter is 12 nm and the molar absorptivity is 1.09*10^8<br.> | ||
*Using Beers Law and the reference above the calculated concentration of the diluted sample is 0.834nM<br.> | |||
*The final concentration of the solution is 8.34nM | |||
Revision as of 18:44, 31 August 2013
 Biomaterials Design Lab Biomaterials Design Lab
|
<html><img src="/images/9/94/Report.png" border="0" /></html> Main project page Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> |
ObjectiveTo prepare gold nanoparticles using two separate methods. Procedure taken from Dr. Matt Hartings, found here. ProcedureBSA-AuNPStock Solutions
Amount of Stock Solution Used
In order to have 90 times less BSA than gold we need 2.82*10^5 milli moles of BSA.<br.>
Citrate-AuNPStock Solutions
Amount of Stock Solution Used
Procedure was followed as written to completion. <br.> After Synthesis of Gold Nanoparticles
UV-Vis Measurements were taken with a sample of solution that was a 10X dilution. As a result, final concentrations found must be augmented to fit this change. Data
In order to analyze the data the following reference was utilized, 'Determination of Size and Concentration of Gold Nanoparticles from UV/vis Spectra'<br.> In order to find the concentration of the solution the Absorbance at 45onm needs to be known.<br.>
|
|