User:Alexander Cvitan/Notebook/Experimental Biological Chemistry Lab/2014/04/02: Difference between revisions
From OpenWetWare
| Line 13: | Line 13: | ||
===Conductivity Measurement of Pure Variables at Room Temperature=== | ===Conductivity Measurement of Pure Variables at Room Temperature=== | ||
*Water was added to sample in order to increase the volume above 11ml. This was done because 11ml of solution was needed in order for the detector to measure the conductivity of the sample. | |||
*Please note that these conductivity values are not corrected for their dilution factor. The true conductivity of these solutions must be further back-calculated. | *Please note that these conductivity values are not corrected for their dilution factor. The true conductivity of these solutions must be further back-calculated. | ||
*Note that MES and 2,2 Bipyridine have reduced conductivity values. This is because other variables are ions, thus further increase this measurement. This may overwhelm observed effects. | *Note that MES and 2,2 Bipyridine have reduced conductivity values. This is because other variables are ions, thus further increase this measurement. This may overwhelm observed effects. | ||
Revision as of 15:16, 17 April 2014
 Biomaterials Design Lab: Spring 2014 Biomaterials Design Lab: Spring 2014
|
<html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> |
Objective
ProcedureConductivity Measurement of Pure Variables at Room Temperature
Atomic Absorption PreparationCreating the Gold Stock Solutions
Atomic Absorption Samples Solutions with the following Au:lysozyme ratio at room temperature were run on the AA:
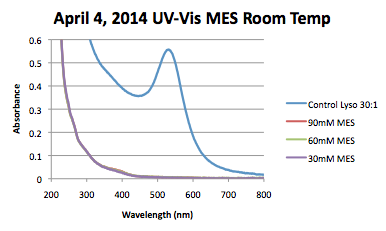
Figures
UV-VIS
| |