User:Alicia Rasines Mazo/Notebook/CHEM-581 Experimental Chemistry I/2014/09/10: Difference between revisions
From OpenWetWare
No edit summary |
No edit summary |
||
| Line 94: | Line 94: | ||
** Bottom curve inflection point: 68.50 ºC<br.> | ** Bottom curve inflection point: 68.50 ºC<br.> | ||
'''PVA-Clay film in 200 ppm MG solution'''<br.> | '''PVA-Clay film in 200 ppm MG solution'''<br.> | ||
[[Image:PVACLay200ppm.png]]<br.> | |||
* Onset of water evaporation: 99.70ºC | |||
* Heat of hydrate decomposition: 1138 J/g | |||
* Mass of water evaporated = 0.00177 g | |||
* Major peaks in water: | |||
** Minimum at 108ºC, other peaks at 110, 131ºC | |||
* Glass transition: | |||
** Top curve inflection point: 68.54ºC | |||
** Bottom curve inflection point: 67.97 ºC | |||
<!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | ||
|} | |} | ||
__NOTOC__ | __NOTOC__ | ||
Revision as of 09:02, 24 September 2014
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |
Tasks for September 10
Completion of Film SynthesisContinued from Dr. Harting's procedure
Film preparation of ionic liquid modified clay
DSC (Differential Scanning Calorimetry) DataNote: To calculate mass of water evaporated the following equation was used: q=mHv. So that mass of water=(q×mass of sample)/Hv where Hv=2.23×103 J/g<br.>
PVA film in 2 ppm MG solution<br.>
PVA film in 8 ppm MG solution<br.>
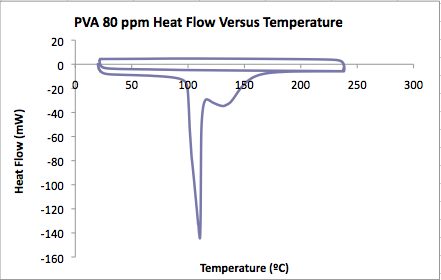
PVA film in 80 ppm MG solution<br.>
PVA film in 200 ppm MG solution<br.>
PVA-Clay film in 2 ppm MG solution<br.>
PVA-Clay film in 8 ppm MG solution<br.>
PVA-Clay film in 80 ppm MG solution<br.>
PVA-Clay film in 200 ppm MG solution<br.>
| |