User:Andy Maloney/Introduction to kinesin and microtubules
Purpose
This page describes what kinesin and microtubules are. It gives a general discussion of why it is important to study this system.
This is the introduction to my completely open notebook science dissertation. If you would like to post questions, comments, or concerns, please join the wiki and post comments to the talk page. If you do not want to join the wiki and would still like to comment, feel free to email me by using the provided link below.
- Click here to join the wiki.
- Click here to email me.
- Click here to post general comments about the open dissertation.
- Click here to post comments to this chapter's talk page.
A pdf version of the Introduction can be downloaded here.
Acknowledgements
I would like to thank Dr. Haiqing Liu for supplying kinesin to our lab and Dr. Gabriel A. Montano. I would also like to thank Dr. Susan Atlas and the support from DTRA CB Basic Research Program under Grant No. HDTRA1-09-1-008 and the UNM IGERT on Integrating Nanotechnology with Cell Biology and Neuroscience NSF Grant DGE-0549500. Finally, I'd like to thank Dr. Erik Schaeffer for his discussions on temperature stabilization.
Introduction
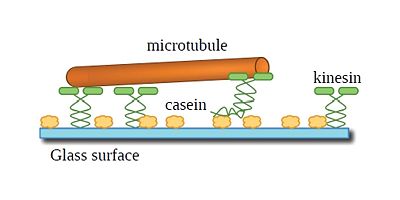
|
| Figure 1: Graphical depiction of a gliding motility assay. The yellow blobs are casein, the green molecules are kinesin and the orange cylinder is a microtubule. The drawing is not to scale. |
Kinesin-1, hereafter called kinesin, and microtubules are cellular components that are vital for life. Kinesin is a motor protein that shuttles cargo from one area of a cell to another. It does so by traveling along microtubules. Microtubules can be thought of as roads designed to allow motor proteins to undergo active transport in a cell.
In this chapter, I will discuss what a kinesin molecule is and why we study it. I will also introduce what microtubules are, and what they are made up of. In Chapter 1, I will discuss how to design and implement a gliding motility assay in order to investigate the kinesin and microtubule system. Gliding motility assays can be thought of as microtubule crowd surfing on a layer of kinesin, see Figure 1. Figure 1 also depicts generally how kinesin is supported by the passivation layer of casein. Chapter 2 discusses observations that indicate that the kinesin and microtubule system is affected by the type of passivation used. Chapter 2 also discusses the importance of temperature stabilization and how without it, obtaining stable gliding speeds are not achievable.
Figure 1 does not show the water molecules that are in the system when investigating the gliding motility assay. If I was to depict the water molecules in this picture, then you would not be able to see any of the proteins since there would be a lot of water molecule obscuring the proteins. Water is an important component in biomolecular interactions and plays a significant role in the microtubule and kinesin system. Chapter 3 discusses the results of some experiments I did that involved changing the isotope of water and measuring gliding motility speeds. It also discusses future experimental work that will allow further investigation to the importance of water interactions in the kinesin and microtubule experiment.
Finally, Chapter 4 discusses open science. This dissertation and all the notebook entries associated with its research was done openly and in the public domain. I discuss my experiences with open science and some success stories from participating in open science.
Kinesin
Kinesin is a dimeric motor protein that can be thought of as having four distinct areas to the molecule, see Figure 2. Figure 2 is a cartoon of a kinesin molecule and is also Koch Lab's kinesin mascot called Kiney. The first major component to the kinesin molecule are the motor domains also known as the heads. The motor domains are depicted as Kiney's feet. They are where chemical energy (in the form of ATP) is converted to mechanical work through hydrolysis. This statement is a very interesting one which is not fully understood and is a major area of study with the kinesin molecule (Cross 2000, Coppin 1997, Kikkawa 2001).
Kinesin walks along a microtubule (Carter 2006, Vale 2000) in much the same way humans walk: we place one foot in front of the other in order to take a step. The difference between how we walk and how kinesin manages to walk along a microtubule is that we have a brain that sends signals to our legs in order for them to take a step. Kinesin does not have a central processing center and thus needs a different method for communication between the two motor domains to signal steps. This communication comes from the linkage of the two motor domains via the motor linker which is also known in the literature as the neck linker. The motor linker can be thought of as a stretchy polymer of amino acids that connects the two motor domains together. The stretchiness of the polymer is a key component to how kinesin sends signals to each of the motor domains (Guydosh 2006, Yildiz 2008). The trailing motor domain of a kinesin takes a step by first docking the motor linker, see Figure 3. This docking results in strain along the motor linker that is a signal for the leading motor domain that the trailing motor is going to take a step. These steps are gated by nucleotide presence and nucleotide states within the motor (Herskowitz 2010). Along with each step is the exclusion of water between the motor domain and the microtubule. I will discuss this in greater detail in Chapter 3.
Figure 2 shows the stalk or coiled coil region of kinesin as Kiney's torso. This is where the two protein chains of kinesin coil around each other and terminate in the cargo binding domain depicted as Kiney's eyebrows. Kinesin is a motor protein that transports cellular cargo (Duncan 2006, Muresan 2000, Nakata 2003). It travels along microtubules in one direction taking 8 nm steps (Svoboda 1993) using one ATP molecule per step (Coy 1999). Kinesin actively transports cargo from one area of the cell to another and can do so by traveling along the microtubule at about 1 μm/s (Herskowitz 2010). This is essential for cellular function. For instance, sciatic nerves can be nearly a meter long. All components generated in cells are done in their nucleus. If diffusion was the only form of transportation for these items, it would take thousands of years for proteins made in the nucleus to be transported to the end of the nerve a meter away. If kinesin were to transport the same items, it would take about 2 weeks.
If the kinesin microtubule system is affected by mutations in kinesin, then this can lead to a variety of diseases (Goldstein 2001, Hurd 1996, Goshima 2003). One such disease causes loss of motor function due to accumulation of cellular cargo transported by kinesin which is lethal. Other mutations that affect humans include Alzheimer's disease (Stokin 2005). Also, the kinesin and microtubule system has been suggested to be used as components in microdevices. Some work has been done to incorporate kinesin and microtubules in devices (Moorjani 2003, van den Heuvel 2005, Korten 2008).
There are several types of kinesin molecules as well as many different types of motor proteins (Sack 1999). This thesis attempts to illuminate experimental procedures necessary in order to obtain good signal to noise data in order to investigate the kinesin and microtubule system.
Microtubules
Microtubules are one type of road produced in cells that kinesin and other motor proteins walk on (Kis 2002). They are polymers made up of subunits called tubulin. Tubulin in turn is made up of two different proteins called α-tubulin and β-tubulin. α-tubulin and β-tubulin combine together to form a single tubulin subunit via a GTP molecule. The bond between α-tubulin and β-tubulin is fairly robust (Desai 1997).
A very interesting thing about microtubules is that they are "polarized" with a "plus" and a "minus" end. These definitions do not refer to the charge of the microtubule. What this terminology is referring to is how a microtubule polymerizes. Free tubulin heterodimers will oligomerize into small segments of a protofilament such that the subunits of tubulin line up in a regular fashion, i.e. (α-β)-(α-β)-(α-β)- etc. This is done through GTP hydrolysis. These oligomers will then polymerize into a microtubule (Mozziconacci 2008). The regularity of how tubulin stacks together "polarizes" the microtubule with an end that polymerizes quickly (the plus end, beta tubulin end) and an end that polymerizes more slowly (the minus end, the alpha tubulin end). This polarization definition is important because kinesin "walks" along the microtubule from the minus to plus end and takes steps on the β-tubulin subunit. The bond between tubulin subunits is not as strong as the bond between the α and β-tubulin subunits. This allows the microtubule to undergo depolymerization when the cell needs to reduce the length of a microtubule. In order to prevent depolymerization, I use a chemical called Taxol to stabilize the tubulin-tubulin interactions (Nogales 1995, Salmon 1984). A brief description of Taxol is given in Chapter 1.
Microtubules are hollow and have an outer diameter of about 25 nm and an inner diameter of 15 nm (Mozziconacci 2008). One thing to note about Figure 4 is that all the protofilaments in the microtubule are lined up in nice neat rows. This is not the case in nature nor is it the case when I polymerize microtubules in a thermal cycler. There does exist some helicity to the protofilaments and it turns out that one can change the number of protofilaments, and thus the helicity, by changing the chemicals that the tubulin is polymerized in. When cells polymerize microtubules, they predominantly have 13 protofilaments. A protein produced in the cells called doublecortin is used to regulate microtubules protofilaments (Moores 2004). When I polymerize microtubules in glycerol, studies have shown that the predominant number of protofilaments is 14 (Ray 1993). I have not measured the number of microtubule protofilaments from the microtubules that I have polymerized. In my experiments, I have seen microtubules vary in length from 2 μm to 40 μm.
Without microtubules, kinesin would have no structure on which to perform active transport. They are essential to cellular structure as well as for cellular replication (Hyman 1987).
Gliding motility assay
The experimental procedure used in this dissertation is the gliding motility assay. Figure 1 shows a basic depiction of what the gliding motility assay is. A glass substrate is first passivated with a protein called casein. I will discuss in depth what casein is and its importance in Chapter 2. For now, it is a layer of protein on the glass that is necessary in order to make a gliding motility assay work. Kinesin, the green entities, are then added to the assay. Kinesin orients itself on the casein such that its motor domains are pointing into solution. The motor domains then attach to a microtubule in solution and walk along it. This propels the microtubule in solution and is similar to how crowd surfing looks. I then visualize the motion of the microtubules through fluorescence.
In the above description, I did not explicitly mention the water that both the kinesin and microtubules are in. Water plays an important role in biomolecular interactions and I will discuss some of the experiments conducted to understand how these interactions occur in this system in Chapter 3.
Conclusion
Kinesin and microtubules are essential for biological functions. Understanding the mechanochemistry of how kinesin hydrolyzes ATP in order to do work is a broad question. It requires the reproduction of consistent experimental tools in order to obtain data that is of sufficient quality. In Chapter 1, I discuss in great detail the experimental procedures required to produce consistent samples of kinesin and microtubules. Producing consistent tools should be the most important thing to an experimentalist and I will discuss this in greater detail in Chapter 1.
Return to the table of contents.
References
- Carter, N. J., & Cross, R. A. (2006). Kinesinʼs moonwalk. Current opinion in cell biology, 18(1), 61-7. doi: 10.1016/j.ceb.2005.12.009.
- Coppin, C. M. (1997). The load dependence of kinesinʼs mechanical cycle. Proceedings of the National Academy of Sciences, 94(16), 8539-8544. doi: 10.1073/pnas.94.16.8539.
- Coy, D. L. (1999). Kinesin Takes One 8-nm Step for Each ATP That It Hydrolyzes. Journal of Biological Chemistry, 274(6), 3667-3671. doi: 10.1074/jbc.274.6.3667.
- Cross, R. A., Crevel, I., Carter, N. J., Alonso, M. C., Hirose, K., & Amos, L. A. (2000). The conformational cycle of kinesin. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 355(1396), 459-64. doi: 10.1098/rstb.2000.0587.
- Desai, A., & Mitchison, T. J. (1997). Microtubule polymerization dynamics. Annual review of cell and developmental biology, 13, 83-117. doi: 10.1146/annurev.cellbio.13.1.83.
- Duncan, J. E., & Goldstein, L. S. B. (2006). The genetics of axonal transport and axonal transport disorders. PLoS genetics, 2(9), e124. doi: 10.1371/journal.pgen.0020124.
- Goldstein, L. S. (2001). Kinesin molecular motors: transport pathways, receptors, and human disease. Proceedings of the National Academy of Sciences of the United States of America, 98(13), 6999-7003. doi: 10.1073/pnas.111145298.
- Goshima, G., & Vale, R. D. (2003). The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. The Journal of cell biology, 162(6), 1003-16. doi: 10.1083/jcb.200303022.
- Guydosh, N. R., & Block, S. M. (2006). Backsteps induced by nucleotide analogs suggest the front head of kinesin is gated by strain. Proceedings of the National Academy of Sciences of the United States of America, 103(21), 8054-9. doi: 10.1073/pnas.0600931103.
- Herskowitz, L. J. (2010). Kinetic and Statistical Mechanical Modeling of DNA Unzipping and Kinesin Mechanochemistry. Thesis.
- Hurd, D. D., & Saxton, W. M. (1996). Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics, 144(3), 1075-85.
- Hyman, A. A., & White, J. G. (1987). Determination of Cell Division Axes in the Early Embryogenesis of Caenorhabditis elegans. The Journal of Cell Biology, 105(11), 2123-2135. doi: 10.1083/jcb.105.5.2123.
- Kikkawa, M., Sablin, E. P., Okada, Y., Yajima, H., Fletterick, R. J., & Hirokawa, N. (2001). Switch-based mechanism of kinesin motors. Nature, 411(6836), 439-45. doi: 10.1038/35078000.
- Kis, A., Kasas, S., Babić, B., Kulik, A., Benoît, W., Briggs, G., et al. (2002). Nanomechanics of Microtubules. Physical Review Letters, 89(24), 1-4. doi: 10.1103/PhysRevLett.89.248101.
- Korten, T., & Diez, S. (2008). Setting up roadblocks for kinesin-1: mechanism for the selective speed control of cargo carrying microtubules. Lab on a chip, 8(9), 1441-7. doi: 10.1039/b803585g.
- Moores, C. A., Perderiset, M., Francis, F., Chelly, J., Houdusse, A., & Milligan, R. A. (2004). Mechanism of microtubule stabilization by doublecortin. Molecular cell, 14(6), 833-9. doi: 10.1016/j.molcel.2004.06.009.
- Moorjani, S. G., Jia, L., Jackson, T. N., & Hancock, W. O. (2003). Lithographically patterned channels spatially segregate kinesin motor activity and effectively guide microtubule movements. Nano Letters, 3(5), 633-637. doi: 10.1021/nl034001b.
- Mozziconacci, J., Sandblad, L., Wachsmuth, M., Brunner, D., & Karsenti, E. (2008). Tubulin dimers oligomerize before their incorporation into microtubules. PloS One, 3(11), e3821. doi: 10.1371/journal.pone.0003821.
- Muresan, V. (2000). One axon, many kinesins: Whatʼs the logic? Journal of neurocytology, 29(11-12), 799-818. doi: 10.1023/A:1010943424272.
- Nakata, T., & Hirokawa, N. (2003). Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. The Journal of cell biology, 162(6), 1045-55. doi: 10.1083/jcb.200302175.
- Nogales, E., Wolf, S. G., Khant, I. A., Luduena, R. F., & Downing, K. H. (1995). Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature, 375(6530), 424-7. doi: 10.1038/375424a0.
- Ray, S., Meyhöfer, E., Milligan, R. A., & Howard, J. (1993). Kinesin follows the microtubuleʼs protofilament axis. The Journal of Cell Biology, 121(5), 1083-1093. doi: 10.1083/jcb.121.5.1083.
- Sack, S., Kull, F. J., & Mandelkow, E. (1999). Motor proteins of the kinesin family. Structures, variations, and nucleotide binding sites. European journal of biochemistry / FEBS, 262(1), 1-11. doi: 10.1046/j.1432-1327.1999.00341.x.
- Salmon, E. D., & Wolniak, S. M. (1984). Taxol stabilization of mitotic spindle microtubules: analysis using calcium-induced depolymerization. Cell motility, 4(3), 155-167.
- Stokin, G. B., Lillo, C., Falzone, T. L., Brusch, R. G., Rockenstein, E., Mount, S. L., et al. (2005). Axonopathy and transport deficits early in the pathogenesis of Alzheimerʼs disease. Science (New York, N.Y.), 307(5713), 1282-8. doi: 10.1126/science.1105681.
- Svoboda, K., Schmidt, C. F., Schnapp, B. J., & Block, S. M. (1993). Direct observation of kinesin stepping by optical trapping interferometry. Nature, 365(6448), 721-7. doi: 10.1038/365721a0.
- Vale, R. D., & Milligan, R. a. (2000). The way things move: looking under the hood of molecular motor proteins. Science (New York, N.Y.), 288(5463), 88-95. doi: 10.1126/science.288.5463.88.
- van den Heuvel, M. G. L., Butcher, C. T., Lemay, S. G., Diez, S., & Dekker, C. (2005). Electrical docking of microtubules for kinesin-driven motility in nanostructures. Nano letters, 5(2), 235-41. doi: 10.1021/nl048291n.
- Yildiz, A., Tomishige, M., Gennerich, A., & Vale, R. D. (2008). Intramolecular strain coordinates kinesin stepping behavior along microtubules. Cell, 134(6), 1030-41. doi: 10.1016/j.cell.2008.07.018.


