User:Anthony Salvagno/Notebook/Research/2009/11/04/Redoing Unzipping: Ligating Anchor, Adapter, pBR322
Setup
{{#widget:Google Spreadsheet |key=tq8wMUU4814eEPtWd16qYlQ |width=500 |height=300 }} I am opting to do one reaction instead of a little trial run. Since I have enough DNA I can do this. Pray to the molecular biology gods that this works. If this works I will make tribute to the gods.
- 30' - added 2ul adapter
- 60' - added 2ul adapter
- 90' - added 2ul adapter
- wait 30 extra minutes after final addition
Gel Results
As you can see there is no 3rd band above the other two. How sad. Koch gave me a list of fixes, which I will list below.
Troubleshooting Ligation
This list is in no particular order but is numbered anyways:
- Check rest of sequences and mfold them
- We checked the sequences and everything matches up. I just need to mfold to see if I get proper duplexing.
- Order new Top and Bottom oligos
- Consider diagnostic oligos (no biotin)
- Steve Koch 22:02, 4 November 2009 (EST): The idea here was to buy a pair of oligos that would have two BstXI overhangs, and thus could create 2.2 kB concatemers out of one 1.1 kB BstXI-digested PCR product.
- Steve Koch 22:03, 4 November 2009 (EST): And actually another idea would be to switch the PCR to something like a 1.6 kB-ish product. That way you could see the BstXI cutting when gel extracting. This may be a pretty good idea, considering we always gel extract anyway.
- Test thermocycler
- I will ask Brian to help out with this one
- Try ligation without hotlid (see below)
Ligation Retry No Hotlid
{{#widget:Google Spreadsheet |key=t22m2uIXtWWwVDBjcWL2cOg |width=500 |height=300 }} I'm trying without the hotlid on, which hopefully was my problem before.
- 20' - added 0.5ul adapter
- 40' - added 0.5ul adapter
- 60' - added 0.5ul adapter
- wait 20' more
Gel Results 2
Sigh... Steve Koch 22:05, 4 November 2009 (EST): Bummer! Note: unless you have a leaking emergency (like this morning), definitely always put in a ladder.
mFold (Dinamelt)
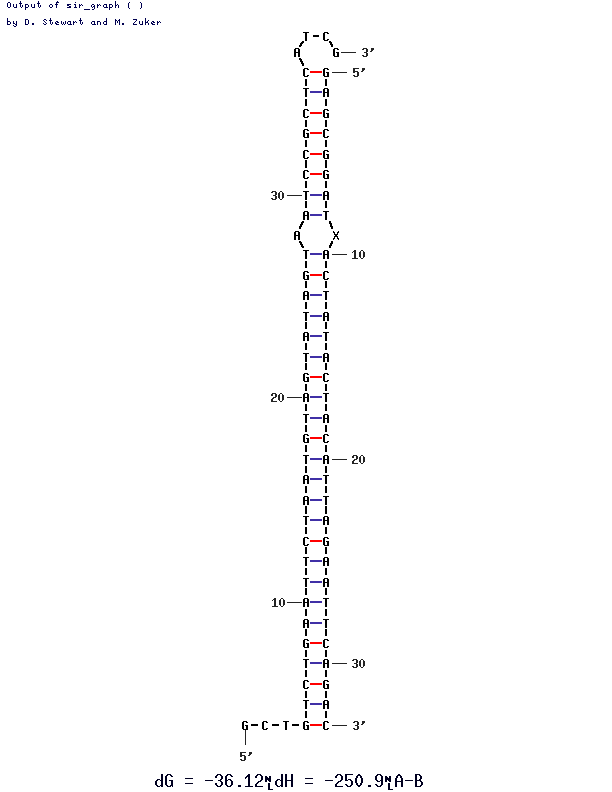
As you can see the two oligos sequences found here (want Top Adapter BstXI/SapI and Bottom Adapter 1a - biotin) match up perfectly. There is a little bubble and that is because I left the X from the bottom adapter sequence in. It should be a T which base pairs with the A. There are the two overhangs as well. My parameters I put in 1M Na+ 0M Mg++ and left it at 37C with .00001M oligo concentrations. If it basepairs under those conditions why not otherwise.
Steve Double-Check
Steve Koch 13:18, 5 November 2009 (EST): I don't know if these links will stay around. Will give it a try to see:
- Top-Ant, Bottom Biotin-Ant (BTW: I'm not sure if we should use this naming. If it's the same as what we ordered before and is on our sequence page, then we should stick with that. Or, come up with another system that provides info. I like the primer numbering I came up with (so you can tell where it's supposed to bind and on what plasmid)
- This pair looks good to me
- Bottom Biotin-Ant, BstXI adapter
- This is not correct! Both overhangs need to be 5 Also, I think you were shooting for the EarI overhang, but we want the 4 basepair BstXI overhang.
- Anthony Salvagno 10:45, 6 November 2009 (EST):Yea I realized this yesterday but did not have time to fix it cause of the Nanocafe. That also is the reason I had no notebook entry (because of class as well). I just couldn't get anything done. I will fix today though.

