User:Ariel Michelle Drew/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| Line 246: | Line 246: | ||
| 14||1%: 11 2%:10||1%: 9; 2%: 0||1%:2 2%: 10||||All fully hatched||1%: Rather curved to the left, and dark pigmentation||1%: large and black, without the hole that the control fish have; 2%: NA||1%: Very curved to the left, much darker pigmentation than control fish, roung "stomach" area below the neck ||All 2% fish found dead and curled up, deformed, gray and floating. All dead fish were thrown out | | 14||1%: 11 2%:10||1%: 9; 2%: 0||1%:2 2%: 10||||All fully hatched||1%: Rather curved to the left, and dark pigmentation||1%: large and black, without the hole that the control fish have; 2%: NA||1%: Very curved to the left, much darker pigmentation than control fish, roung "stomach" area below the neck ||All 2% fish found dead and curled up, deformed, gray and floating. All dead fish were thrown out | ||
|} | |} | ||
''Experimental Group'' | |||
'''Introduction''' | '''Introduction''' | ||
Latest revision as of 19:20, 26 March 2015
Lab 1-- Observing a Niche at AU: Wildlife Preserve 1/14/15
Introduction/Purpose: This procedure is attempting to examine the various forms of organisms across the American University campus. Organisms are dependent on the environment and thus certain Niches will be more favorable for particular organisms. I must assert that if the Wildlife Preserve Niche contains quite a bit of disrupting factors, as well as favorable ones (lots of direct sunlight, and precipitation) then there will be a median amount of diversity within the the medium sized population of organisms.
Materials and Methods: This procedure required paper and pencil for observation, and a 50 mL plastic container as well as abiotic and biotic factors (listed below) of the Niche for sample collection. The sample was kept at room temperature after the Hay Infusion (requiring 11 grams of vegetative sample, 500 mLs of deerpark water, 0.1 gm dried milk—mixed together for 10 seconds) was completed. Then the jar was labeled and the top was removed. Next, the open jar was placed near a window.
Data and Observations:
Niche appears to get copious amounts of light and precipitation. Niche is thick with biotic features such as trees (one containing red berries, the other with no leaves), moss, grass, bushes, and a taller more distinctive form of grass. Niche is considerable polluted with litter, but also contains more favorable biotic factors such as rocks, benches, dirt, and mulch (distinctive from the dirt observed). It is also important to note that the Niche appears to be unprotected from wind, and contains quite a bit of foot traffic.


Conclusions and Future Directions: I predict that Niches in direct sunlight, and that contain large amounts of precipitation, and relatively few disrupting factors (such as wind and foot traffic) will have more copious and diverse forms of life. Considering the forceful wind observed as well as the large amount of litter noticed in the Niche, it could then be concluded that this Niche contains quite a bit of disrupting factors, thus inhibiting a larger organism population. I am not yet certain whether this will affect the diversity in organisms observed. Next time I may want to harvest soil samples from 5 inches below the surface as well as the surface to test if the foot traffic affects organism population and diversity.
AD
Lab 2-- Hay Infusion Culture Observations and Preparing and Plating Serial Dilutions-1/21/15
Introduction/Purpose: The purpose of this experiment was to establish what types of microorganisms live in a particular niche (in this case the wildlife preserve niche) and possibly determine a carrying capacity (the maximum number of individuals of a species that can survive in a particular niche), and prepare the organism samples for lab next week. If our niche contains plentiful microbiotic specimens then I assert that they will be rather singular in structure.
Materials and Methods: The Hay Infusion Culture Observations procedure required a pipet to extract samples from the Hay Infusion (kept at room temperature) and a slide to put those samples on. A microscope was used to observe the organisms, while a dichotomous key is used to identify. The Preparing and Plating Serial Dilutions procedure required four tubes of 10 mLs sterile broth labeled with 10^-2, 10^-4, 10^-6, 10^-8. A micropippetor set at 100 microliters with tips was also needed. Four nutrient agar and four nutrient agar plus tetracycline plates were obtained. Plates from each of the two groups were labled 10^-3, 10^-5, 10^-7, 10^-9. 100 microliters from the Hay Infusion culture was added to the 10 mLs of broth in the tube labeled 10^-2 and mixed thoroughly, making it a 1:100 dilution. 100 microliters of broth from tube 10^-2 is added to tube 10^-4 and mixed thoroughly (making a dilution of 1:10,000). This process is repeated two more times to make the 10^-6 and 10^-8 dilutions. To plate serial dilutions on the nutrient agar plates, pipette 100 microliters from the 10^-2 tube onto the nutrient agar plate 10^-3. This procedure is repeated with the 10^-4 dilution onto the 10^-5 plates, the 10^-6 dilution onto the 10^-7 plates and the 10^-8 dilution onto the 10^-9 plates. Agar plates are then placed side up in a rack where they will incubate at room temperature for one week.
Data and Observations: • Smell: Earthy, stale, swampy. Appearance: No apparent life on top. Coated in a goopy, crusted grey-purple slime on top. Organisms in Top Sample: Colpidium (motile via cilia) (about 50-70 micrometers long) (protozoa because it is a motile, unicellular, eukaryotic organism). Paramecium Caudatum (motile via cilia) (about 200-300 micrometers long) (protozoa because it is motile, unicellular eukaryote). Volvox (motile via flagella) (about 200 micrometers in diameter) (Type of Green Algae). Organisms in Bottom Sample: Colpidium (no other organisms were noted, despite the observation of several samples from the bottom of the culture). No photosynthesizing organisms were noted. • The Volvox organism obtains energy through conversion to ATP by way of photosynthesis. The Volvox forms a colony of up to 50,000 cells. The individual Volvox cells are connected to each other by thin strands of cytoplasm, this allows the colony to swim as a coordinated unit. Each colony is formed with an exterior ball of swimming cells, and an interior ball of reproducing cells. In this colony of 50,000 cells, there is no nervous system. Swimming thus cannot be conducted by way of electrochemical nervous system signals, so most likely swimming is achieved by crowd-sourced behavior. Volvox is a form of algae, and this type of algae is attracted to light so that photosynthesis may be achieved, and thus this crowd-sourced movement will be drawn towards light. Volvox is a asexual organism, containing both somatic cells (which do not reproduce) and gonidia near the posterior, which produce new colonies through repeated division. Ancestors of Volvox transitioned from single cells to multicellular colonies at least 200 million years ago, with this transitory period lasting approximately 35 million years. • If the Hay Infusion Culture “grew” for another two months, I would expect the amount of protists and algae to have significantly increased. I would assume that amount of sunlight available would be a significant selective pressure on the community of the sample. It would also be reasonable to assume that certain portions of the Culture will receive more sunlight than others, and depending on whether organisms are motile or not, whether they can reach these better lit sections of the Culture.
Table 1:
| Nutrient Solution | Nutrient Solution | Nutrient Solution |
| 10^-2 | 10^-3 | 10^-3 |
| 10^-4 | 10^-5 | 10^-5 |
| 10^-6 | 10^-7 | 10^-7 |
| 10^-8 | 10^-9 | 10^-9 |
Conclusions and Future Directions: Our samples seemed relatively lifeless, and found organisms were rather similar in structure. While our niche did not appear to support a plethora of organisms, I was correct in assuming there would be relatively little diversity among the organisms found. Perhaps this lack of life, and singularity is a result of sampling error. If I were to redo the experiment I would collect samples for the hay infusion culture that were about half a foot below the surface to ensure an untainted sample from the plethora of foot traffic and trash present. I would also study more slides of the Hay Infusion culture to test whether life varies based on where the sample is taken from (top, or bottom) (left or right) (middle). I would also like to test whether temperature has any affect on the types of organisms collected.
References: Bentley, Meg; Walters-Conte, Kathryn; Zeller, Nancy. 2015. A Laboratory Manual to Accompany: General Biology 210. Department of Biology, American University. American University; Washington DC. 12-24.
Wim, van Egmond. "Volvox, One of the Seven Wonders of the Micro World." Microscopy UK. December 2003. Micscape Magazine. 1/19/15.
AD
Lab 3-- Microbiology and Identifying Bacteria with DNA Sequences – 1/28/14
1) Archaea tends to prefer extreme environments, while the agar plates do not present these conditions they also do not inhibit such growth. 2) The sample has turned into a dark, muddy brown color, with a deep brown crusted encasing the top of the sample. Overall the culture has developed a rather potent rotten earth odor. If the sample is cultivating the mass division of microorganisms, then this increase in bacteria (and fungi?) could cause the altering appearance and smell.
Procedure 1: Identifying and Studying Bacteria
Table 1: 100-fold Serial Dilutions Results
| Dilution | Agar Type | Colonies Counted | Conversion Factor | Colonies/mL |
| 10^-3 | Nutrient | 0 | ×10^3 | 0 |
| 10^-5 | Nutrient | 25 | ×10^5 | 2500000 |
| 10^-7 | Nutrient | 132 | ×10^7 | 1320000000 |
| 10^-9 | Nutrient | 60 | ×10^9 | 60000000000 |
| 10^-3 | Nutrient+ TET | 0 | ×10^3 | 0 |
| 10^-5 | Nutrient+ TET | 0 | ×10^5 | 0 |
| 10^-7 | Nutrient+ TET | 0 | ×10^7 | 0 |
| 10^-9 | Nutrient+ TET | 50 | ×10^9 | 50000000000 |
Procedure 2: Antibiotic Resistance
1) The plates with nutrient present visible colonies and fungi, while the plates with the antibiotic (TET) do not—TET plate 10^-9 is the exception, thus indicating that such growth is either due to antibiotic resistant bacteria or human error through contamination of plates. In general the TET appears to decrease if not eliminate all bacteria and fungi on the plates where it is introduced. 2) The mechanisms of action for tetracycline is for it to eliminate gram positive and gram negative bacteria, richettsia, spirochetes, and large viruses. Tetracycline is often revered as successful in riding the body of Escherichia coli, Haemophilus Influenzae, Mycobacterium tuberculosis, and Pseudomonas aeruginosa.
Klan, Rafal. “Antimicrobial Properties”. Chemistry and Chemical Biology of Tetracycline. 2015. Institute of Organic Chemistry, PAN. (2/2/15) http://www.chm.bris.ac.uk/motm/tetracycline/tetracycline.htm
Procedure 3: Bacterial Cell Morphology Observations
Table 2: Bacteria Characterization
| Colony Label | Plant Type | Colony Description | Cell Description | Gram + or Gram - | Additional Notes |
| 4 | With TET | Sarcina, 1 mm | No flagella, non motile, circular | Gram + | N/A |
| 3 | With TET | N/A | N/A | N/A | Possibly washed off when gram staining or sample posses no life |
| 4 | Without TET | Hypha, 5mm, light pink | Cilia, motile | Gram - | N/A |
| 3 | Without TET | Sarcina, light purple, ½ - 1 mm | No flagella, non motile, circular | Gram + | N/A |
AD
Lab 4-- Plantae and Fungi
Introduction:
The purpose of this lab was for our group to identify five plant samples from the Wildlife transect and categorize these samples based on three factors: 1) Presence of Vascularization: 2) Presence of Specialized Structures: 3) Mechanisms of Reproduction.
Materials and Methods:
Two Ziploc bags were used to collect and contain samples from the transect. Using a scalpel, the stems of the leaves were cut at the tip and put under the microscope to identify the vascular system of that particular leaf.
Data and Observations:
| Transect Sample Plants | Location and Number in Transect | Description, Size and Shape | Vascularization | Specialized Structures | Mechanisms of Reproduction |
| #1 Leaves at the Base of the Central Tree | Leaves on the Ground Near the Base of the Large, Central Tree | 7 1/2 Inches in Length, 3 Inches in Width. Long, Narrow Leaf with Parallel Lines | Vascular, bundles are scattered. | Seeds | Gymnosperm |
| #2 Leaves at the Base of the Small Tree | Leaves on the Ground Near the Base of the Small Tree On the Precipus of the Transect | 5 1/2 Inches in Length, 5 1/2 Inches in Width. Broad Leaf with a Network of Viens. | Vascular, bundles are in rings and scattered. | Seeds | Gymnosperm |
| #3 Moss on Stones around Central Tree | Moss Grows in Patches on Stepping Stones that Surround the Central Tree | 1/2 Inch in Width | Non-Vascular | Rhizoids | Bryophyte |
| #4 Small Bushes | Small Bushes Encase the Peripheries of the Transect | 8 Inches in Length, 3 Inches in Width. Long, Semi-Broad Leaf with a Network of Viens. | Vascular, bundles are in rings and scattered. | Seeds | Gymnosperm |
| #5 Tall Bushes | Tall Bushes Stand in the Back of the Left Corner of the Transect, Behind the Bench | 3 Inches in Length, 1 Inch in Width. Short and Broad Leaf with Parallel Lines | Vascular, bundles are scattered. | Seeds | Gymnosperm |
Conclusion:
There were no flowering plants in the transect and so we were unable to identify monocot or dicot seeds. Fungi Sporangia can be multicellular or unicellular and contain an enclosure of spores. All plants and fungi form spores at one point in their life cycle. Sporangia can produce spores by mitosis, but in nearly all land plants and many fungi, sporangia are the site of meiosis and produce genetically distinct haploid spores.
Work Cited
"Definition of Sporangium in English:." Sporangium. N.p., n.d. Web. 13 Feb. 2015.
Rost, Barbour, Stocking, Murphy, 2006Plant Biology, 2nd EditionThompson Brooks/Cole
AD
Lab 5-- Invertebrates Lab
Introduction: The Purpose of this lab was to identify invertebrates from a sample collected from the transect, and understand how simple systems have evolved into more complex systems.
Materials and Methods: For this lab our group broke down the Berlese Funnel and gently pour the top 10-15 mLs of liquid and organisms into one petri dish. The remaining liquid was the poured into a second dish. Then both dishes were examined using a microscope and invertebrates are identified.
Data and Observations: The Dugesia flatworm (also know as a planarian) is a acoelmate with no blood vascular system, making this worm anatomically simple that relies on diffusion for all its transport needs. A notable anatomical feature of this worm is the ciliated epidermis, a centralized brain with nerve cords extending throughout the rest of the body, various muscle fibers responsible for movement. Though this worms possess a simplified anatomy, the planarian exhinits certain complex behaviors. Planarians revel a negative phototaxis behavioral response, meaning that they evade light.
The nematode contains a simple structure of an internal body cavity called a pseudocoelom, and the lack of features such as cilia and a well-defined head that are found in most animals. The cuticle is the closest thing a roundworm has to a skeleton, and in fact the worm uses its cuticle as a support and leverage point for movement. Long muscles lie just underneath the epidermis. These muscles are all aligned longitudinally along the inside of the body, so the nematode can only bend its body from side to side, not crawl or lift itself. A free-swimming roundworm thus looks rather like it is thrashing about aimlessly.
Phylum Annelida have both longitudinal and circular muscle fibers in body wall; peristalsis.¾ special muscles attached to setae to move setae, ¾ in polychaetes, oblique muscle groups run into the parapodia for movement. Peristalsis involving the alternating contractions of longitudinal and circular muscle layers to change the shape of individual segments ¾ burrowing and creeping using peristalsis and/or parapodia.
| Organism (Phylum and Class) | Length in mm | Number in Sample | Description of Organism |
| 1. Springtail X Primitive Insect | 1mm | 1 | Found on the top sample. Contains 6 legs, one tail, and two antlers. The body is long, slender and slightly bent. |
| 2. Thrips | .8mm | 2 | Found on the top sample. Contains 6 legs, and two wings. |
| 3. Soil Mite | .7mm | 4 | Found on bottom sample. |
| 4. Beetle Larva | 2mm | 2 | Found on bottom. |
Conclusion:
Cooper’s Hawk, Phylum: Chordata, Class: Aves, Order: Accipitriformes, Family: Accipitridae, Genus: Accipiter, Species: A. Cooperii. Biotic Factors: Berries from tree could serve as a food source. Abiotic Factors: Debris could act as shrapnel to build nests.
Raccoon, Phylum: Chordata, Class: Mammalia, Order: Carnivora, Family: Procyonidae, Genus: Procyon, Species: P. Lotor. Biotic Factos: Berries from tree and insects/worms from soil could serve as food source, large central tree has many holes that could serve as shelter. Abiotic Factors: Large amounts of degrees and grass protect from being seen by predators.
American Robin, Phylum: Chordata, Class: Aves, Order: Passeriformes, Family: Turdidae, Genus: Turdus, Species: T. Migratorius. Biotic Factors: Berries from tree could serve as a food source. Abiotic Factors: Debris could act as shrapnel to build nests.
Black Squirrel, Phylum: Chordata, Class: Mammalia, Order: Rodentia, Family: Sciuridae, Genus: Sciurus, Species: S. Carolinensis. Biotic Factos: Berries from tree and insects/worms from soil could serve as food source, large central tree has many holes that could serve as shelter. Abiotic Factors: Large amounts of degrees and grass protect from being seen by predators.
Gray Rat, Phylum: Chordata, Class: Mammalia, Order: Rodentia, Family: Rattus, Species: R. Norvegicus. Biotic Factos: Berries from tree and insects/worms from soil could serve as food source, large central tree has many holes that could serve as shelter. Abiotic Factors: Large amounts of degrees and grass protect from being seen by predators.
The cohabitation of these species living in the Wildlife Preserve Transect at the same time forms a community within this transect. These species are dependent upon one another for food and maintaining a stable environment. By predator species and prey species living together in this environment, population remains stable and thus will not surpass the niche’s carrying capacity. There is are only so many environmental resources to ensure the survival of a given number of species, and only so many prey options to provide a food source to the predators of the niche. Furthermore, there are only so many available shelter options in this niche. Thus due to limited resources for both the prey and the predator, carrying capacity is never breached. The trophic levels established in this environment only further solidifies the kinds of organisms that will prosper and how many will survive. The population must remain low enough to sustain primary producers such as plants, which in turn will sustain herbivores, which will in turn sustain the predators of this niche.
Work Cited
Aggarwall, S. (n.d.). A Study of Planarian Phototaxis, Tactile Response and Regeneration. Retrieved February 23, 2015.
Introduction to the Nematoda. (n.d.). Retrieved February 28, 2015.
Phylum Annelida. (n.d.). Retrieved February 23, 2015
AD
Lab 3 Revisited-- PCR and 16s Sequencing
During Lab 3 of bacteria studies, samples of colonies were taken from two TET plates and two non-TET plates. PCR products on an agarose gel were then run, and the DNA of the PCR product that showed the 16s gene were then purified for sequencing. Once these samples were sequenced, the result was used to identify the bacteria in our transect. This identification was done using the nucleotide BLAST system, which contains the DNA sequences of all known organisms-- the system matches your input sequence to the closest match(es) in its database.
Our first bacteria had a 98% match with Flavobacteria. This organism is known to be a motile, rod-shaped, gram-negative bacteria that favors moist soil or fresh water. The second bacteria had a 98% match with Epilithonimonas. This organism is know to be a non-motile, rod shaped, gram-negative bacteria that favors moist soil or costal water.
AD
Zebra Fish Observations
| Day | Total Fish | Living Fish | Dead Fish | Random Size | Stages | Tails Description | Eyes Description | Body Description | Notes |
| 1 | 20 | 20 | 0 | all in stage A (zygote) | NA | NA | Transluscent Eggs, visible embryos | NA | |
| 4 | 20 | 20 | 0 | 45 (4x) | 4 stage A (zygote); 1 stage B (fertillized); 1 stage E (thirty two); 1 stage A (256); 1 stage E (dome); 1 stage A (2-somite); 2 stage J (15-somites); 4 stage L (17-somites) | NA | few apparent, pale | Almost completely translucent and cloudy, no embryos observed | 100% Eggs have a much more translucent/cloudy appearance as compared to the control |
| 7 | 1%: 10 2%:10 100%: 20 | 1%: 10 2%: 10 100%: 0 | 100%: 20 | 1%:approximately 48-60hrs 2%: approximately 48-60hrs 100%: Clear, empty eggs | 1%: Straight, short 2%: Straight, short | 1% and 2%: Dark pigmentation | 1% and 2%: Straight, short, egg sac still slightly attached | All 100% fish eggs sterilizedby salt and thrown away, 1% and 2% groups were made | |
| 11 | 1%: 10 2%:10 | 1%: 10 2%: 10 | 0 | All fully hatched | 1% and 2%: Curving slightly to the left, darker color | 1% and 2%: Dark Pigmentation | 1% and 2%: Curving slightly to the left, darker pigmentation | 1% and 2% appear to be responding similarly to the salt environment | |
| 14 | 1%: 11 2%:10 | 1%: 9; 2%: 0 | 1%:2 2%: 10 | All fully hatched | 1%: Rather curved to the left, and dark pigmentation | 1%: large and black, without the hole that the control fish have; 2%: NA | 1%: Very curved to the left, much darker pigmentation than control fish, roung "stomach" area below the neck | All 2% fish found dead and curled up, deformed, gray and floating. All dead fish were thrown out |
Experimental Group
Introduction
The purpose of this experiment was to study the effects of different concentrations of salt (1%, 2%, and 100%) on the development of zebra fish for a period of 14 days, with a control set aside for comparisons.I hypothesize that in the salt containing solutions, less zebra fish maturation will occur—resulting in organism deformities—and that in the potent salt concentrations (2% and above) the zebra fish will fail to survive, seeing as zebra fish are tropical freshwater fish (Sawant, Zhang, & Li, 2001).
Material & Methods
Day 1 The control group and 100% saline solution experimental group were established. 20 healthy zebra fish embryos were set up in 20mLs of Deerpark water in a petri dish labeled as the control. 20 more healthy zebra fish embryos were set up in 20mLs of 100% saline solution in a petri dish labeled as the 100% saline experimental group. Zebra fish embryos were determined as healthy if they were translucent and did not contain a coating of fuzz. A dropper was used to transfer the embryos to from the zebra fish container to the two respective petri dishes. Using a dissection microscope, observations on the average developmental stages of each group were approximated.
Day 4 The zebra fish in both groups were observed using the dissection microscope to determine the number of dead eggs, the number of living embryos still in egg cases, the number of living hatchlings, and the number of dead hatchlings. Using a micropipette set at 20mLs and separate tips, 10mLs of water or test solution and any waste were removed and 25mLs of fresh Deerpark water or fresh test solution were added to the control group. Using a dropper any waste was removed from the experimental group.
Day 7 The zebra fish in the experimental group and control group were observed using the dissection microscope to determine the number of dead eggs, the number of living embryos still in egg cases, the number of living hatchlings, and the number of dead hatchlings. 5mLs of water was removed from the control group with any egg cases and 5mLs of fresh Deerpark water was added using a micropipette set at 20mLs. On this day the 1% and 2% saline experimental groups were created. To create a 1% test solution, 0.2mLs of saline solution was added --using a micropipette and micropipette tips-- to 19.8mLs of Deerpark water, combined in a labeled petri dish. To create a 2% test solution, 0.4mLs of saline solution was added –again using a micropipette and micropipette tips—to 19.6mLs of Deerpark water, combined in a labeled petri dish. Each experimental group then had 10 recently hatched zebra fish transferred into the respective petri dishes by way of a dropper. One drop of food (Paramecium) was added to the two experimental groups and the one control group.
Day 11 The zebra fish in the two experimental groups and control group were observed using a dissection microscope to determine the number of live zebra fish and the number of dead zebra fish in each group. The degree of body, eye, and tail pigmentation and shape were also noted along with any observations on abnormal movement. In the control, 5mLs of water and any waster were removed, and 10mLs of fresh Deerpark water were added via a micropipette and micropipette tips. In the experimental groups, 5mLs of solution and any waste were removed, and 10mLs of water+ saline solution were added to each group. One drop of Paramecium was then added to the two experimental groups and the one control group.
Day 14 Final observations on zebra fish were made, using a dissecting microscope, to determine the number of live zebra fish, the number of dead zebra fish and any appearance observations such as body, eye, and tail pigmentation and deformities in shape. Two dead experimental zebra fish from the 1% saline solution were added to a tube with 3mLs of water, one drop of tricaine solution per mL of water, and paraformaldehyde. This developmental slide fix was stored for future references.
Results
On day 4, the control contained approximately 20 healthy zebra fish embryos at stage 14—Somite (“Zebra Fish Facility,” 2012), contained in a 100% solution of Deerpark water. The original experimental group contained approximately 20 fertilized zebra fish eggs—approximately between the “1k-cell” stage and the “high” stage (“Zebra Fish Facility,” 2012)— contained in a 100% saline mixture. It was difficult to determine a stage for the experimental group, as the embryo was very cloudy.
On day 7, all 100% saline group embryos were noted as extraordinarily clear and void of life, and were consequently thrown away. Before the disposal, pictures of the apparently empty or sterilized embryos were attempted but did not show up on camera. At this point two new experimental groups were formed: a 1% saline solution group, and a 2% saline solution group. Each new group contained 10 recently hatched zebra fish. The zebra fish were approximated to be between 48-60 hours old (“Zebra Fish Facility,” 2012) as their tails, while mostly straight, were short, and the body still had the egg sac attached at the diaphragm region. The control group was approximated to be a comparable developmental stage for similar reasons. A developmental slide fix should have been made on this day with three zebra fish from the test group, however due to the fact that all the zebra fish in the 100% test group died, this process was negated.
On day 11, the 1% and 2% saline test groups are both noted as curving slightly to the left, both in body and tail, and as having a darker pigmentation, in eyes, tail, and body. However, despite these slight alterations, there does not appear to be much difference in appearance between the control and experimental groups, and almost no difference between the 1% and 2% test groups. An experimental error was committed here, proper documentation through body size measurements and pictures were not taken of the deformed specimens in the 1% and 2% test groups. In the control group, one mutated zebra fish was noted; no eyes were apparent, and the head was indistinguishable from the abdomen. A developmental slide fix was recommended on this day, however due to the few number of zebra fish in each test group, this process was again negated.
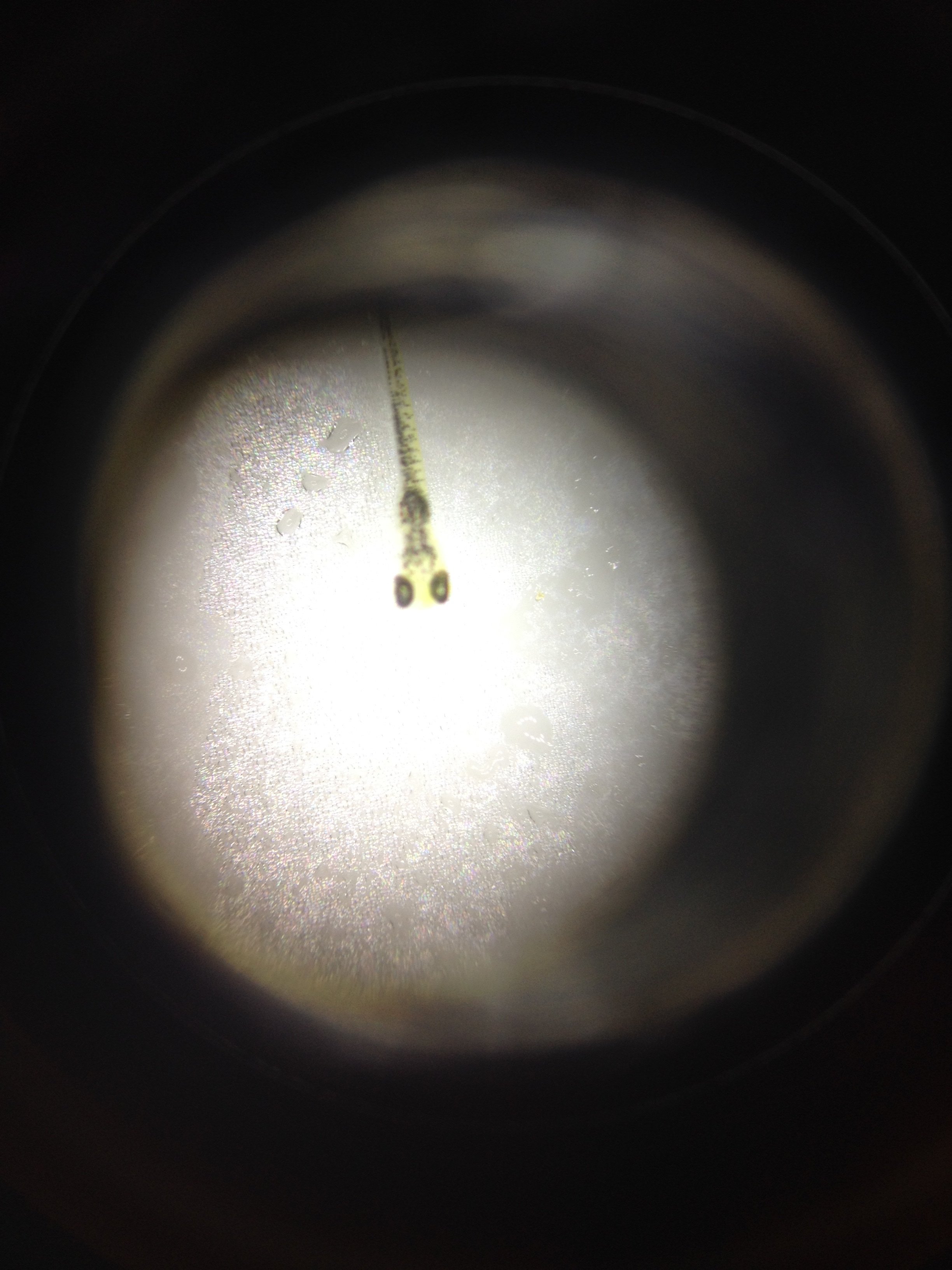
On day 14, all 2% zebra fish were found dead and curled up, pigmented a deep grey, and severely deformed and misshapen with extreme left curvature noted in the body and tail. Some dead 1% zebra fish were found in the same manner, but not to the magnitude that was observed in the 2% test group. The live 1% zebra fish held the same deformity and discoloration as noted in the dead 2%, and were observed to be much less mobile as compared to the live control zebra fish. Furthermore, the 1% test group’s eyes were much larger and darker than the control. The body length of the dead zebra fish noted were approximated to be 115um on a 4x objective, while the dead zebra fish were approximated to be 130-140um on a 4x objective. It is important to note that a data collection error or possible experimental error was made here; 11 zebra fish—both dead and alive—were noted in the 1% petri dish on day 14, while a total of 10 zebra fish were observed in the 1% petri dish on day 11. On this day a developmental slide fix was created with dead zebra fish from the 1% test group, one of the zebra fish used in this slide fix.
Discussion
Due to the eye and tail deformities and the overall smaller size of the 1% test group (as compared to the control), it would appear that an increased presence of salt in the environment of the developing zebra fish, negatively impacted the zebra fish growth rate and yielded strong, negative effects on the development of certain features. However, the exact degree to which an increasingly salty environment negatively impacts the zebra fish remains unclear due to inadequate observation and measurement methods of the 2% test group. Furthermore, the attempt of observing a 100% saline group was ill planned and should have been totally negated. It would have been much more productive to have originally started with a 1% test group and a 2% test group, instead of adding in these two groups when all of the 100% test group eggs were sterilized by the harsh, salty environment.