User:Cosette Brianna Taggart/Notebook/Biology 210 at AU: Difference between revisions
| Line 1: | Line 1: | ||
==Lab Write Ups== | ==Lab Write Ups== | ||
'''March 4, 2015''' -- CT | |||
23-- >MB92-Rev_16S_G03.ab1 | |||
NNNNNNNNNNNNNANNNNANNGCGCCNTNCNNGCAGCTACTNNNNNNNACTTCTGGAGAGACGGCTCGACGGGTGNGGTG | |||
TGCGNGNGTAGGAGACCCGTCANCGTATTCTTGGTGACATTCTGATACAAGATTACCAGCGATCCCGACTTCNAGTTGTC | |||
GACTTGCATACTGCCAGCCAGAGGACTACAGGGATTGTGGGATTAGCTCCCCCTCGCGGGTTGGCAACCCTNTGTACCAN | |||
CCATTGGAGGACGTGTGGAGCCCCGGCCATAAAGGACATGAGGACTTGACCTCCTCCCCCCCTTCCTCCGGTTTGTCACC | |||
GGTAGTCCCCTTANAGTGCCCAACTGAACGTAGCAACTAATGGGGAGGGTTGGTTGCGTTGCTTGACTTAACCCCCCATC | |||
TCACGACATGANCAGACGACTGCCACGCCGCATCTGTGGGCTGGTAGGCTTTCAAGGACCCAACCCTCTTTGGTAACTTT | |||
CTGCCCTGGGAAAGGTGGGTAAGGTTTTTCCCGTTTAATCCAAATAAACCACATCATGCGCCGGTTGTGGGGGTCCCCGT | |||
TGATTCTCTTTATTTTCAACCTTGTCGCCGTGCTCCCCACGTGGTGCATTTTATNCGATACTTTCNGTACTGAGTCANCT | |||
NAGACCCATCANCCTTTTGACATNGNTTAAGGAGGGGANNANCNCGGGNTCTATTCCNGCTTTNTCCCCNCNCTTTNNCG | |||
CNAGAACNTCANTGAGCTGCCNNGGNANTGCCNTCTCCATCAGTGATCCNCNCNNTATATACCCACTTGTCTGATTCNGC | |||
CGGAATTNCATCCCCCCCCTGCCCCACNNTATCCTTGCNNTGCNATGGTNGAGCCCCCGTNGATCNCCNNNANTTTNANN | |||
NTNTNNNNANNCACCCCNGCGCNCGCTATATNCNCATAAATTTNAAATANNCNTATTCCCNCNNCTTTTNNGCNGGGNGN | |||
NGGGNNAANNNTNANNCTGTNCTTATTNTTNNNNNNNNNNNNNNANNNNGGGNTATTNNNGNNNNNCNTCTNNTTNCNNN | |||
NNNANNACANTAAAANNNCANNNCCNCGNCNGCNNNNGCTTTGCTGNTTGNNNNNTTTNNNNNNNNNNNACANNNCGCNN | |||
NN | |||
Uncultured Comamonas sp. clone 127 16S ribosomal RNA gene, partial sequence | |||
24-- >MB93-Rev_16S_H03.ab1 | |||
NNNNNNNNNNNNNNCNNANNNNNCTNNNGTTACGCGTCACCGACTATCNAGTACCCGGTACTTCCNTGGCTTGGCGGGCG | |||
GTGTGTACAGGGCCCGGGAACGTATTCACCGCGCCATGGCTGATGCGCGATTACTATCGATTCCAACTTCAAATAGTCGA | |||
GTTGCAGACTCCAATCCGAACTGAGACCAGCTTTCGATATTTGGGATTGGCTCCTGTGTAGCTGCCCTCTGTACTGGCCA | |||
TTGTATTACGTGTGAGGCGCGAGGCCTAAGGGCCGTGGTGATTTAACATCTTCCCCACCTTCCTCTCTACTTGCGTATGC | |||
CGTCTCACTACACTCCCCAACTTAATGATGGAAACTATTGAGAGGGGGTGCGCTCGTTGCGCGACTTAACCTAACACCTC | |||
ACGGCACGAGCTGACAACAACCATGCGCCACCTTGGAAAATGTCCNAACAAGAGTCTATTTCTAAACCTGTCATTTCCCA | |||
TTTANGCCTTGGGGAGGTTCCTCGCGTATCNNCGAATTAAACCACATAATCCACCGCTNGTGCGGGGCCCCGTCNATTCC | |||
TTTGANTTTCATTCTTGNNNNNGTACTCCCCACGTGGCTNACTTATCACTTTCNCTTANTCTCTGAATCCNAANATCNAA | |||
AAACNAGTTANCATCGTTTACAGCGTGNACTACCAGGNNATCTAATCNTGTTCGCTCNCCACGCTCTCNTCCATCANCGT | |||
CANNNGTTGCTTAGTAACCTGCCNTCGCAATTNNNGTTCTNAGTAATATCTATGCATTTCACCGCTACNCTACNTATTCC | |||
CGCTACTTCAACAACACTCAANACCTGCANTATCAATGGCAGTTTNANANTTAANCTGTGAGATTTCACCACTGACTTAT | |||
NTATCCNCCTACAGACCCTGTANACNCTATANATCCNGANNANNCTAGCACCNTCCGCANTACCNCTACTGCTGGNACGG | |||
AGTTNGCNNNGTGCNTATTCNTATANTNNNTTCAGCTANCATNCACNTANCTNGGTNNNTNCCCTATNNANNNNNNTTTA | |||
NGACCNATAGGGNCNNCGNCCNTCANNCNNNATGGCTGGATCAGGNTNGCANNCATTGNGCANTANNCCNNNNNTNNNGA | |||
NNNNNNN | |||
Chryseobacterium sp. JCM 10535 gene for 16S ribosomal RNA, partial sequence | |||
'''February 18, 2015''' -- CT | '''February 18, 2015''' -- CT | ||
Revision as of 23:03, 3 March 2015
Lab Write Ups
March 4, 2015 -- CT
23-- >MB92-Rev_16S_G03.ab1 NNNNNNNNNNNNNANNNNANNGCGCCNTNCNNGCAGCTACTNNNNNNNACTTCTGGAGAGACGGCTCGACGGGTGNGGTG TGCGNGNGTAGGAGACCCGTCANCGTATTCTTGGTGACATTCTGATACAAGATTACCAGCGATCCCGACTTCNAGTTGTC GACTTGCATACTGCCAGCCAGAGGACTACAGGGATTGTGGGATTAGCTCCCCCTCGCGGGTTGGCAACCCTNTGTACCAN CCATTGGAGGACGTGTGGAGCCCCGGCCATAAAGGACATGAGGACTTGACCTCCTCCCCCCCTTCCTCCGGTTTGTCACC GGTAGTCCCCTTANAGTGCCCAACTGAACGTAGCAACTAATGGGGAGGGTTGGTTGCGTTGCTTGACTTAACCCCCCATC TCACGACATGANCAGACGACTGCCACGCCGCATCTGTGGGCTGGTAGGCTTTCAAGGACCCAACCCTCTTTGGTAACTTT CTGCCCTGGGAAAGGTGGGTAAGGTTTTTCCCGTTTAATCCAAATAAACCACATCATGCGCCGGTTGTGGGGGTCCCCGT TGATTCTCTTTATTTTCAACCTTGTCGCCGTGCTCCCCACGTGGTGCATTTTATNCGATACTTTCNGTACTGAGTCANCT NAGACCCATCANCCTTTTGACATNGNTTAAGGAGGGGANNANCNCGGGNTCTATTCCNGCTTTNTCCCCNCNCTTTNNCG CNAGAACNTCANTGAGCTGCCNNGGNANTGCCNTCTCCATCAGTGATCCNCNCNNTATATACCCACTTGTCTGATTCNGC CGGAATTNCATCCCCCCCCTGCCCCACNNTATCCTTGCNNTGCNATGGTNGAGCCCCCGTNGATCNCCNNNANTTTNANN NTNTNNNNANNCACCCCNGCGCNCGCTATATNCNCATAAATTTNAAATANNCNTATTCCCNCNNCTTTTNNGCNGGGNGN NGGGNNAANNNTNANNCTGTNCTTATTNTTNNNNNNNNNNNNNNANNNNGGGNTATTNNNGNNNNNCNTCTNNTTNCNNN NNNANNACANTAAAANNNCANNNCCNCGNCNGCNNNNGCTTTGCTGNTTGNNNNNTTTNNNNNNNNNNNACANNNCGCNN NN
Uncultured Comamonas sp. clone 127 16S ribosomal RNA gene, partial sequence
24-- >MB93-Rev_16S_H03.ab1 NNNNNNNNNNNNNNCNNANNNNNCTNNNGTTACGCGTCACCGACTATCNAGTACCCGGTACTTCCNTGGCTTGGCGGGCG GTGTGTACAGGGCCCGGGAACGTATTCACCGCGCCATGGCTGATGCGCGATTACTATCGATTCCAACTTCAAATAGTCGA GTTGCAGACTCCAATCCGAACTGAGACCAGCTTTCGATATTTGGGATTGGCTCCTGTGTAGCTGCCCTCTGTACTGGCCA TTGTATTACGTGTGAGGCGCGAGGCCTAAGGGCCGTGGTGATTTAACATCTTCCCCACCTTCCTCTCTACTTGCGTATGC CGTCTCACTACACTCCCCAACTTAATGATGGAAACTATTGAGAGGGGGTGCGCTCGTTGCGCGACTTAACCTAACACCTC ACGGCACGAGCTGACAACAACCATGCGCCACCTTGGAAAATGTCCNAACAAGAGTCTATTTCTAAACCTGTCATTTCCCA TTTANGCCTTGGGGAGGTTCCTCGCGTATCNNCGAATTAAACCACATAATCCACCGCTNGTGCGGGGCCCCGTCNATTCC TTTGANTTTCATTCTTGNNNNNGTACTCCCCACGTGGCTNACTTATCACTTTCNCTTANTCTCTGAATCCNAANATCNAA AAACNAGTTANCATCGTTTACAGCGTGNACTACCAGGNNATCTAATCNTGTTCGCTCNCCACGCTCTCNTCCATCANCGT CANNNGTTGCTTAGTAACCTGCCNTCGCAATTNNNGTTCTNAGTAATATCTATGCATTTCACCGCTACNCTACNTATTCC CGCTACTTCAACAACACTCAANACCTGCANTATCAATGGCAGTTTNANANTTAANCTGTGAGATTTCACCACTGACTTAT NTATCCNCCTACAGACCCTGTANACNCTATANATCCNGANNANNCTAGCACCNTCCGCANTACCNCTACTGCTGGNACGG AGTTNGCNNNGTGCNTATTCNTATANTNNNTTCAGCTANCATNCACNTANCTNGGTNNNTNCCCTATNNANNNNNNTTTA NGACCNATAGGGNCNNCGNCCNTCANNCNNNATGGCTGGATCAGGNTNGCANNCATTGNGCANTANNCCNNNNNTNNNGA NNNNNNN
Chryseobacterium sp. JCM 10535 gene for 16S ribosomal RNA, partial sequence
February 18, 2015 -- CT
~Identifying Invertebrates~
Introduction -In this experiment the lab explored different types of invertebrates within each group's niche. Before the lab began 3 types of worms were observed for movement; these were Planaria, Nematodes, amd Annelida. Planaria move in a smooth gliding motion over surfaces, Nematodes move by contacting and stretching their bodies, and Annelida move in the same way that nematodes do. The purpose of this weeks experiment was to understand the importance of invertebrates and observe the invertebrates that collected in the Berlese Funnel over the past week.
Materials and Methods -In this weeks experiment Berlese Funnels, made in the previous lab, were collected and the invertebrates that had collected were observed. To do so, the Berlese Funnel was broken apart and the top 10-15 mLs were poured into one petri dish and the remaining liquid was poured into another. To identify the organisms within the liquid an online dichotomous was used along with a microscope to aid in the view of the organisms. The findings were then recorded in a table.
Data
-In the data shown below 2 Arthropoda Arachnida and 3 Arthropoda Insecta were observed. They ranged in size from 75-160mm @ 4X.

Conclusion - In conclusion, this experiment showed the diversity of invertebrates that are contained within just 1 given niche on campus. There are plenty of other species that may live in this niche. For example vertebrates that may visit the community garden niche are Didelphis virginiana (opossum), Apodemus sylvaticus (Field mouse), Rattus rattus (black rat), Mimus polyglottos (mockingbird), and Turdus migratorius (Robin). In the next lab different substances will be given to zebrafish embryos to observe the developmental process.
February 11, 2015 -- CT
~Identifying Plantae and Fungi~
Introduction - Plants are broken down into 2 groups Bryophytes, non-vascular, and Tracheophytes, vascular (Bentley, 2015). Plants are an essential part of ecology. In this experiment the lab class explored different the different plant life within a given niche around AU campus.The purpose of this week's experiment is to understand the characteristics and diversity of plants as well as appreciate the function and importance of fungi.
Materials and Methods - In this experiment 5 different plant species were taken from a selected niche. Also, ~500g of leaf litter was collected for next weeks experiment. Both of these were brought back to the lab. Many different types of samples were then observed under microscope. The collected plant samples were then taken and observed for description, vascularization, specialized stuctures, and mechanisms of reproduction. Then, samples of fungi were observed. Lastly, a Berlese Funnel was then made to collect invertebrates.
Conclusion -In conclusion, this experiment showed the diversity of plants that grow within just 1 given niche on campus. After review of data is was found that #1 was of the Brassica genus, #2 the Zea genus, #3 the Thymus genus, #4 Brassica genus, and #5 remained unknown. When the fungi were observed you could see the sporangia which are important to the fungi reproduction. The second image above is a rendering of the fungi observed which was a zygomycota. To further understand this niche/transect the Berlese Funnel will be studied the next lab for invertebrates.
February 4, 2015 -- CT
~Identifying Bacteria~
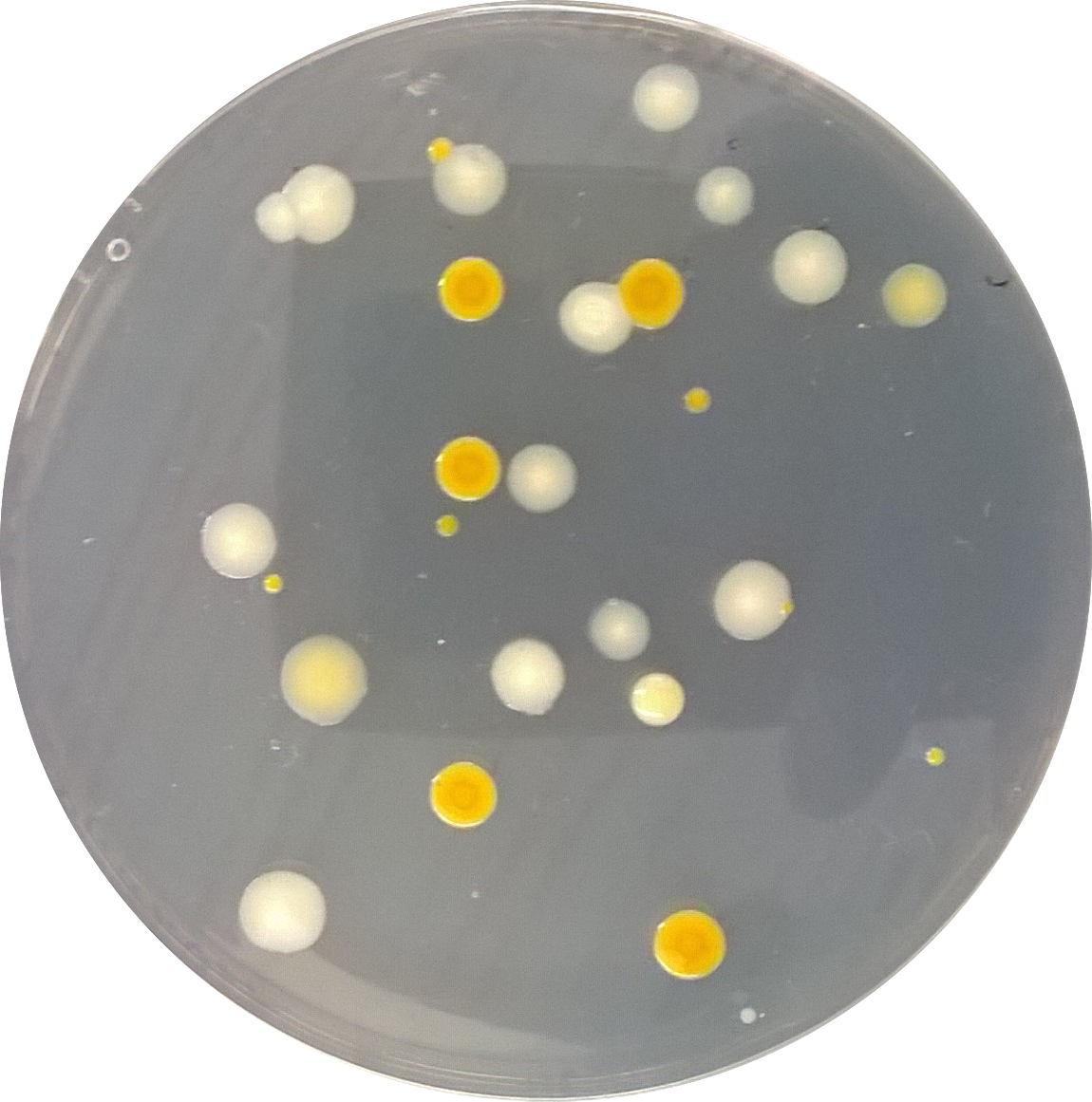
Introduction -Bacteria is one of 3 domains of life which consists of proteobacteria, chlamydaie, sprirochetes, actinobacteria, firmicutes, and cyanobacteria (Bentley, 2015). Bacteria have 3 basic shapes bacillus, coccus, and spirillum (Bentley, 2015). The purpose of this week's experiment is to observe and identify different bacteria growning in serial dilution plates of nutrient and tetracycline plates, created in the previous week. Although some Archaea may be growing on the tetracycline plates because they contain antibiotic this is because archaea stereotypically grow in harsh/extreme environments.
Materials & Methods -In this class the Hay-infusion container, made 2 weeks ago, was observed for one last time. After, the serial dilution plates were collected and observed for the number of colonies and general description. Samples of colonies were taken and plated onto a slide with a drop of water to be observed under a microscope. Then, a gram stain procedure was then done on some plates.
Data - When the hay infusion was observed a hypothesis that was made about why the appearance and smell my change from week to week was: As the hay infusion continues to grow new populations of organisms and decay others then the appearance and smell would change. There were a few differences between the nutrient and the antibiotic plates such as: color of colonies and the amount of overall colonies. This indicates that the antibiotics are able to resist growth of bacteria. When the plates were reviewed under a microscope many type of bacteria were identified such as coccus, streptococci, bacillus filmentous, coccobacillus, club rod, and hypha.


 File:WP 20150128 005.png
File:WP 20150128 005.png




 File:WP 20150128 013.png
File:WP 20150128 013.png
Conclusion -in conclusion, this experiment showed the diversity of bacteria within a given niche (AU garden). Six different species were found within only 2 plates which means that there could have been many many more. To further understand the bacteria the PCR results will be studied in the next lab.
January 28, 2015 -- CT
~Identifying Algae and Protists~
Introduction - Algae and protists are the larger groups of unicellular eukaryotes, organisms that have membrane bound nuclei and organelles (Bentley, 2015). Algae is a eukaryote that photosynthesizes and protists are eukaryotes that consume/hunt nutrients to survive (Bentley, 2015). The purpose of this week's experiment is to observe and identify algae/protists in each groups Hay-infusion, created in the previous week. There is a multitude of different types of algae/protists which a tool called a dichotomous key can be used to facilitate the identification of each (Bentley, 2015).
Materials & Methods - In this class the Hay-infusion container, made last week, consisting of 10-12 g of soil from the group's niche, 500 mL of water, and 0.1 g of dried milk was collected without being disturbed. The hay infusion (Image 1) was then opened and studied for smell and appearance. After observation of the Hay-infusion 2 samples were taken with a pipette from the top (Image 2) and the bottom (Image 3) of the container. From the samples wet mounts were made to observe what protists and algae were present in each. Drawings and measurements of protists and algae were recorded.
Data -The hay-infusion had a very pungent odor when opened. It was a musty, earthy, and lake-like smell. The hay-infusion in appearance had a translucent mold film with small white round colonies dispersed throughout. The top niche sample from the hay infusion contained paramecium bursaria (Drawing 1) a motile protozoa with photosynthesizing components, spirostomum (Drawing 2) a motile protist, and blepharisma (Drawing 3) a motile protist. The bottom niche sample from the hay infusion contained pandorina (Drawing 4) a non-motile algae, peranema (Drawing 5) a motile protist, paramecium multimicronucleatum (Drawing 6) a motile protozoa, and spirostomum (Drawing 2).
Conclusion
-In conclusion, this experiment showed the diversity of protists and algae within a given niche (AU garden) is very apparent. Six different species were identified within hay-infusion. Pandorina meet all the needs of life described on page 2 of the Freeman text because this organism is able to obtain and use energy though photosynthesis, it is made up of cells, it contains genetic information and it is capable of replication. If the hay-infusion were to grow for another 2 months I would expect to see a increase in mold/algae and possibly a decrease in life. This is because as the protist continue to reproduce and grow they may overpopulate for the nutrients available to them in the infusion container. To further the understanding of this transect serial dilution plates will be studied next lab for prokaryotes and possibly fungi.
January 26, 2015 -- CT
~Biological Life at AU~
Introduction - Ecology is the study of how organisms interact with one another in one environment (Bentley, 2015). In this experiment the lab class explored different niches around AU campus. The purpose of this experiment was to observe an ecosystem of biotic, living, organisms and abiotic, nonliving, components (Bentley, 2015). Both biotic and abiotic features are important to the biodiversity of an ecosystem/ niche. By students familiarizing themselves with their niche, it will be helpful for the upcoming experiments.
Materials & Methods -The class was broken into multiple groups of 3 and assigned a transect. Each group received a tube to collect a soil sample. At arrival of the group's transect, they observed the surroundings for abiotic and biotic factors. They then drew a birds-eye view of their transect. Lastly, the groups collected a soiling sample which the group was to believe most representative of their transect. The soil was brought back to the lab for a Hay-Infusion Culture.
Data - The transect was located behind Leonard Hall in the fenced AU garden/farm. The abiotic factors included: the fence, wooden planters, mulch, rocks and watering hoses. The biotic factors included: grass and plants that were growing in the planters. The transect birds-eye view can be seen below in Figure 1.
Conclusion -This experiment showed one of the many transects on campus which portrays the diverse ecosystem on campus. As stated in the purpose many biotic and abiotic features were noted for such a small area. To further the understanding of this transect a hay infusion will be studied next lab for protists and algae.
References - Bentley, Meg. 2015. A Laboratory Manual to Accompany: General Biology II. American University: Washington, DC. Pg.16-17













