User:Courtney Krawczyk/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
[[Image:16s.jpg]] | [[Image:16s.jpg]] | ||
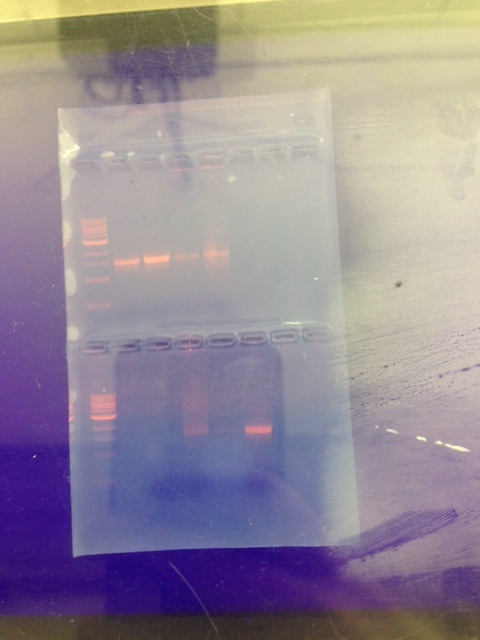
Our samples that were sent out for sequencing are the two top left samples next to the ladder. | |||
Revision as of 16:48, 3 March 2015
DNA sequencing of the 16S Gene March 3, 2015
Purpose The purpose of this experiment was to determine what species of bacteria are living in our transect.
Materials and Methods After running a PCR for the 16S gene, two of our samples were sent out for sequencing. Once this was completed we were able to view our sequence, and then use the NCBI Blast program to determine what species was actually in this transect. Both of our samples being sequenced came from our nutrient agar plats, not the plates with the tetracycline.
Data and Observations
Our samples that were sent out for sequencing are the two top left samples next to the ladder.
Sequence for MB59
NNNNNNNNNNNNNNNNNNAGCNNNGCAGTCGAGCGGANGANGGGAGCTTGCTCCTTGATTCAGCGGCGGACGGGTGAGTA
ATGCCTAGGAATCTGCCTGGTAGTGGGGGACAACGTTTCGAAAGGAACGCTAATACCGCATACGTCCTACGGGAGAAAGC
AGGGGACCTTCGGGCCTTGCGCTATCAGATGAGCCTAGGTCGGATTAGCTAGTTGGTGGGGTAATGGCTCACCAAGGCGA
CGATCCGTAACTGGTCTGAGAGGATGATCAGTCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTG
GGGAATATTGGACAATGGGCGAAAGCCTGATCCAGCCATGCCGCGTGTGTGAAGAAGGTCTTCGGATTGTAAAGCACTTT
AAGTTGGGAGGAAGGGCAGTAAGCTAATACCTTGCTGTTTTGACGTTACCGACAGAATAAGCACCGGCTAACTCTGTGCC
ANCANCCGCGGTAATACAGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCGCGTANNTGGTTTGTTNNGTT
NNATGTGAAANCCCCNGGGCTCAACCTGNGGAACTGCATCCCNNACTGGNNNNCTANANTACGGTGNANGGTGNTGNAAT
TTCNTGTGNNGNNNTGAAATGCNTAGATANANCATTAACAGCGGNGACNANGGGACNNCNTNNACTNGTTNGNNCCNCNN
ATGTGCGNNNNCNGGTGNNGNAAACANNNNNGAGNNCCNCCTTCGTCNNNGNNNNNNNNANNATNNNNCTACCCCNTTNN
NNNGNTNNNNGNANTTNCCNNGCCTNNCCNCNNGANNNNANCNNNNCGTTNNNNNAGCNCNCCNTNGNGNNNACNGGNNT
TTNNNAGNCNNNGNNNANNCAACNNNNNNAGNNNNNGTNGNNCNANTNNNNNNAAAANNNATNNNNNNNNCCNTNNNNTA
GNACGNNNGACGNNNNNNNNANNNNGNNNNN
Sequence for MB60 NNNNNNNNNNNGNNNGCTTNNNNTGCAGTCGAGCGGGGAGATGTAGCTTGCTACATTTCCTAGCGGCGGACGGGTGAGTA ATGCTTAGGAATCTGCCTATTAGTGGGGGACAACGTTTCGAAAGGAACGCTAATACCGCATACGCCCTACGGGGGAAAGC AGGGGATCTTCGGACCTTGCGCTAATAGATGAGCCTAAGTCAGATTAGCTAGTTGGTGGGGTAAAGGCCTACCAAGGCGA CGATCTGTAGCGGGTCTGAGAGGATGATCCGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTG GGGAATATTGGACAATGGGGGGAACCCTGATCCAGCCATGCCGCGTGTGTGAAGAAGGCCTTTTGGTTGTAAAGCACTTT AAGCGAGGAGGAGGCTACTAGTACTAATACTACTGGATAGTGGACGTTACTCGCAGAATAAGCACCGGCTAACTCTGTGC CAGCAGCCGCGGTAATACAGAGGGTGCGAGCGTTAATCGGATTTACTGGGCGTAAAGCGTACGTAGGCGGCTTTTTAAGT CGGATGTGAAATCCCTGAGCTTAACTTAGGANTTGCATTCGATACTGGGAAGCTAGAGTATGGGAGAGGATGGTAGAATT CCAGGTGTAGCGGTGAAATGCGTAGAGATCTGGAGGAATACCNATGGCNAANGCGGCCATCTGGCCTGNTACTGACGCTN ANGTACGAAANCATGGGGAGCANACAGGATTAGATACCCTGNTAGTCCATGCCNTANNCNATGTCTACTNNCCNTTGGGG CCNNTGANNNNNTANTNNCNCAGCTCACGCNATAANTNNACCGCCTGGGNAGTGNNGNNCNCAGNNTANACTCAAATGNN TTGACANNGNCCNGCACAANCGNTNGANCATGNGNNTTTNNNTCNATGCANNNNNANNNCCNTNACCTGCTNNTTGNNGN TANNNNNNANCNNNNNNGGNANATNNNNTNGGNGGCGNTNGNTNANCTTNNNGNANANNTNNCNNCNTNGNNNNGNANGN GCTNNNNGTCNNCNNNTGNNNGNNNANNNNNGGNNNAANNNNNNNNNNNNNNNNNNTNNNNNTNNNNNN
MB59 Species- Pseudomonas putida strain YN3 MB60 Species- Acinetobacter bouvetii strain CCM7196
Conclusion The Pseudomonas putida is a gram negative, rod shaped y-probacterium (Ye, L). It has a diverse range of metabolic activities and are able to survive in highly polluted ares (Ye, L). The fact that it was gram negative matched our findings on this bacteria. We saw this bacteria as circular not rod shaped, but this might be because there are different strands of this particular bacteria. The Acinetobacter genus is gram-negative, nonmotile, non-fermenting cocci or coocobaccilli (Kumar, A). It can be isolated from a wide spectrum of environments, including coil, water, plats, humans, insects, and sewage (Kumar, A). These characteristics are similar to our findings for this strain in that they are gram-negative, nonmotile, and circular in shape (similar to how cocci should appear).
References Kumar, A. (2010, November 1). Characterization of a novel trimethoprim resistance gene, dfrA28, in class 1 integron of an oligotrophic Acinetobacter johnsonii strain, MB52, isolated from River ... Retrieved March 3, 2015, from http://www.academia.edu/2347902/Characterization_of_a_novel_trimethoprim_resistance_gene_dfrA28_in_class_1_integron_of_an_oligotrophic_Acinetobacter_johnsonii_strain_MB52_isolated_from_River_
Ye, L. (2014, November 4). Draft Genome Sequence Analysis of a Pseudomonas putida W15Oct28 Strain with Antagonistic Activity to Gram-Positive and Pseudomonas sp. Pathogens. Retrieved March 3, 2015, from http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0110038
CK
'Vertebrates that Inhabit our Transects' February 16, 2015
Purpose The purpose of this study was to consider different vertebrates that might inhabit or pass through our transect. After determining what vertebrates interact with the transect, we were to determine the classification of each. We then were to consider what biotic and abiotic elements in the transect affect these invertebrates.
Materials and Methods To view any vertebrates that may interact with this transect, we walked through our transect and observed the area for a couple of minutes. I went in the late afternoon around 4pm on a cloudy day. We also noted the biotic and abiotic elements in the transect. To create a food web based on the groups of organisms in the transect, we modeled the diagram based on figure 56.4 from the text ().
Data and Observations Although we did not see any vertebrates within our transect, we did come to some conclusions of vertebrates that may pass through the area, based on vertebrates that we saw in the surrounding areas. First, squirrels were visible on campus. Squirrels belong to the Chordata phylum, and these squirrels are probably part of the Sciuridae family (Animal Diversity). Also, what appeared to be chipmunks were seen around this area. Chipmunks are also part of the Chordate phylum (Animal Diversity). Two bird species that may pass through here could be robins, and blue jays. I have seen robins on campus myself, and I know that other lab groups have seen blue jays in the near by areas. Robins are part of the Chordate phylum and this robin specifically is probably known as the T. m. migratorius (Animal Diversity). Blue jays are part o the Chordata phylum, the Corvidae family and the species is known as the C. Cristata (Animal Diversity). Last, rabbits have been seen in the surrounding areas of my transect as well. They are also part of the Chordata phylum, and they are specifically part of the Leporidae family (Animal Diversity). The chipmunks, squirrels and rabbits can feed on grass and the plants within our transect. All of the vertebrates, but specifically birds would probably use sticks and twigs to create nests or their homes. Below you can find a food web based on the organisms I have observed in my transect throughout the semester. These species relate to community in a ecological concept because there is more than one species, making up populations, living in this same geographical area. Although they may not live in this specific transect, they are all passing through and sharing the area. Carrying capacity is related to the maximum size a population can get based on the resources available. Given that many species are sharing the area, it is important that each species is able to obtain the resources they need to survive. Trophic levels are related to the order in the food chain, of which organisms feed off others. The food chain for this transect can be found below.
Conclusions As the transect portion of the lab wraps up, it is clear than many different organisms inhabit such a small transect that is just a tiny part of an entire ecosystem. Everything from fungi to bacteria to plants, invertebrates, and vertebrates use the environment in different ways for survival.
Works Cited
http://animaldiversity.org/accounts/Sciurus/classification/
CK
Observing Invertebrates from our Transect February 16th, 2015
Purpose In this study, we observed invertebrates that we found in our transect that we were able to obtain from of the Berlese Funnels we set up last week. We recorded their body size, how many of each invertebrate was in the sample, a description of their appearances, and was phylum and class they probably belong to. We also viewed acoelomates, pseudocoelomates, and coelomates through the dissecting and compound microscopes, looking specifically at their body structures and movement.
Materials and Methods To view the acoelomates, pseudocoelomates, and coelomates we looked at them using the dissecting and compound microscopes that were previously set up for us before the start of lab. The acoelomate, Planaria were fed egg yolk so the digestion could be viewed. To examine the invertebrates from the berlese funnel, we first had to break down the berlese funnel. We pipetted the top half of the ethanol solution into one petri dish, and the lower second half into another petri dish. We then used the dissecting mircoscopes to find an invertebrates from the sample. After we found an invertebrate, we could prepare a slide with the invertebrate to view in the compound microscope, so we could see the distinct body structures more closely.
Data and Observations
The above chart gives the descriptions of all the invertebrates found in the berlese funnel. The first two in the chart actually came from our berlese funnel, but because we could only find two, we examined invertebrates found in another lab group's funnel, but still from the same transect. The first invertebrate we examined, which we believed to be an insect, was the largest of the five invertebrates we viewed. It was also the invertebrate we found the most of from the transect. (The order of the invertebrates listed in the chart, appear in the same order in the pictures below. Invertebrate 3 and 4 both appear in the third picture). Looking at the acoelomate, Planaria its movement appeared as though it were gliding. The nematodes looked as though they were slimy and slithering around when viewed under the dissecting microscope. The coelomate Annelida also appeared that they had swimming life movement. Below you can find pictures of all 5 invertebrates we found from our transect.
Conclusion Many invertebrates were found within our transect that we did not see with the naked eye, or outside. It took the making of a berlese funnel to even obtain these invertebrates for viewing. The second part to this lab will discuss vertebrates from in this transect.
CK
Observing and Understanding the Characteristics of Plantae and Fungi February 10, 2015
Purpose In this study, we collected 5 different plants from our transect and observed the structures, vascularization, and the mechanisms of reproduction for each plant. We also looked at fungi that had grown in our agar plates under the dissection microscopes and compared it to other samples of fungi set up in the lab. Last, we set up a berlese funnel to collect invertebrates to examine to the next lab class.
Materials and Methods First we went out to our transect (#5) and described five different plants. We noted their location and brought samples of the plants back with us in a plastic bag to the lab. In the lab we observed these plants, and recorded the visible characteristics in an organized chart. After looking at the plants, we looked at different samples of fungi with a dissecting microscope and tried to determine the type of fungi we were looking at. We also then looked at a small sample of fungi that had grown in our agar plates. Finally, we set up the berlese funnel by putting leaves from a leaf litter sample(that we had also collected from our transect) into a funnel that had a a screen taped to the bottom so the leaves could not fall through the funnel. (The leaf liter was a collection of dead leaves, plant matter, and a little soil). The funnel was then set on a ring stand while the bottom was being held in a 50 mL conical tube containing 25 mL of a 50:50 ethanol/water solution. A lighted 40 watt lamp was places above the funnel with an incandescent bulb about 1-2 inches from the top of the leaf litter. This was all covered in foil and left for the week to observe in the following class.
In the table above, all the descriptions for each plant are listed. When we tried to look at the fungi that had grown in our agar plate, it was difficult to see any structures. Therefore, we looked at the samples we set up in class and we believed that we had viewed basidomycota (mushroom), zygomycota, and ascomycota. The fungi sporangia is important because it plays a vital role in fungi reproduction. Below are also pictures of the different plants we had found in our transect.
Conclusions Overall many different kinds of plants exist in our transect. Next week we will view the invertebrates from our transect.
CK
Observing Bacteria and Antibiotic Resistance January 31st, 2015
Purpose The purpose of this experiment was to look at bacteria samples we had grown on nutrient agar plates and nutrient agar plates plus the antibiotic tetracycline taken from our hay infusion culture. We looked for differences between the two plates and observed the characteristics of the colonies that grew. This experiment also included setting up a PCR of the bacteria colonies to later DNA sequence it and be able to identify the species from the sequence. It was hypothesized that the agar plates containing the antibiotic tetracycline would have less bacteria growth that the nutrient agar plates.
Materials and Methods First my lab partners and I picked four specific colonies that we wanted to examine on the microscopes. Two from the nutrient only plates and two from the tetracycline. After selecting and labeling the colonies we would observe, we sterilized a loop scrapper that we would use to scrape the bacteria off the agar plate. From there we made 4 separate wet mount slides, sterilizing the loop with the flame between each scrape. After making the wet mount slides, we then used the same four colonies to make 4 more gram stain slides. To make the gram stain slides, you place a small amount of water onto the slide. Then scrape a small amount of the bacteria with the loop and mix it in the water, just like making a wet mount slide. Next, the water needs to dry on the slide before adding the stain, so the slide needs to be raised above a bunsen burner flame. It was waved back and forth until it dries, without burning the bacteria. After this is complete, over a stain tray, the bacteria smear was covered with crystal violet for about 30 seconds, then rinsed with water. Then it was covered with Gram's iodine mordant for 1 minute, then rinsed again with water. The slides were then decolorized with 95% alcohol for 10-20 second and rinsed again. Finally they were covered with safranin stain for 20-30 seconds and rinsed a final time. The excess liquid was blotted off carefully with a kimwipe. The slides were all now ready to view. The final part of this lab was to set up a PCR for 16s sequencing. This was done for each of the four colonies we previously looked at on the sides. First a single colony was transferred into 100 microliters of water in a sterile tube. It was then incubated at 100 degrees celsius for 10 minutes in a heat block. Next, it was centrifuged for 5minutes at 13,400 rpm. While this was centrifuging, 20 microliters of a primer/water mixture was added to labeled PCR tuves and mixed to dissolve the PCR bead. Finally 5 microliters of the supernatant from out centrifuged sample was transferred to the 16S PCR reaction. This was done for each sample of bacteria, making a total of four separate tubes. The samples were then going to go through PCR while we were not in lab so they would be ready to run on an agrose gel for the next lab.
Data and Observations Before looking at the bacteria, my lab partners and I took more one look at the hay infusion culture before disposing of it. It had a stronger more moldy smell, more growth on top, and some water appeared to have evaporated. It also looked like something may have been growing on the sides of the jar. ext we examined the agar plates and recorded the growth of bacteria colonies on each plate. WE found that the 10^-3 agar nutrient plate had the most growth. It was not even possible to count all of the colonies. THe number than decreased as the dilutions did, with zero colonies found on the 10^-9 nutrient agar plate. The nutrient plus tetracycline plate of 10^-3 was the only plate of the tetracycline plates to grow any colonies at all, with an estimate of about 480 colonies. Overall there was much more bacteria on the plates without tetracycline. The Antibiotic plates also appeared to have bacteria more yellow in color. The nutrient only plates had both white and yellow bacteria. Fungus appeared to have grown on both of the 10^-3 plates, but more so on the nutrient only plates. Tetracycline works by binding to the 30S ribosome of bacteria. This prevents attachment of the tRNA to the RNA-ribosome complex (Mehta, A). Bacterial pathogens such as Chlamydia and Mycoplasma are some types of bacteria that are sensitive to Tetracycline (Chopra, I). When viewing the slides we had made, the first bacteria we looked at was from a nutrient only 10^-7 plate. The colonies appeared circular, and the actual individual cells looked like spirals, possibly streptococci, and they were not motile. They were also gram negative. The next bacteria slide observed was also taken from 10^-7 nutrient only plate. THe colony appeared as a cluster of very small bacteria, circular, and greenish black in color. The individual cells were not motile, circular, and this was also gram negative. The bacteria taken from the tetracycline plate was taken from the 10^-3 dilution plate. The colonies appears as spirals all connected together, possibly filamentous, and were greenish black in color. They were not motile cells and were also gram negative. The last slide (taken from the same tetracycline plate) had colonies that appeared green and purple, they were oddly shaped, possibly irregular or rhizoid. This was the only slide to appear gram positive. Below, the red picture of bacteria was from the first tetracycline colony. The purple bacteria, was taken from the second tetracycline colony, and appeared gram positive.
Conclusions and Future Direction Overall, our hypothesis was correct, in that more bacteria grew on the nutrient only plates than the nutrient plus tetracycline plates. Next week will be able to identify our bacteria based on the DNA sequencing from the PCR.
References Chopra, I., & Roberts, M. (2001, June 1). Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Retrieved January 31, 2015, from http://mmbr.asm.org/content/65/2/232.full
Mehta, Akul (2011-05-27). "Mechanism of Action of Tetracyclines". Pharmaxchange.info. Retrieved 1/31/15.
CK
Observing and Identifying Algae and Protists January 29th, 2015
Purpose The purpose of this study was to observe and identify different organisms from the culture we had made in the previous lab. The second part of this lab consisted of plating serial dilutions of the mixture of bacteria species onto nutrient agar plates to observe at a later time.
Materials and Method First, I took a sample for one area of the hay infusion culture (top right side) and made a wet mount slide. Using the microscope I looked at the slide in search of any organism, then observed and recorded data on what I saw. I then repeated this step, but this time I took a sample from a different part of the hay infusion culture (the bottom left of the jar). After observing and recording this information, I used a dichotomous key to help identify what organisms I may have seen that were present in the culture. The second part of the experiment was setting up agar plates to allow bacteria to divide many times to form colonies and look at on another date. First four nutrient agar plates and four nutrient agar plates plus tetracycline were obtained and labeled either 10^-3, 10^-5, 10^-7, and 10^-9 (each type of plate was labeled with a different dilution). After labeling the plates, the hay infusion culture was swirled and mixed thoroughly. Four different tubes full of 10mLs sterile broth each, were also labeled 10^-2, 10^-4, 10^-6, and 10^-8. Then 100 microliters of the culture was assed to the 10mls of broth in the 10^-2 tube. THen 100 microliters of broth from that tube was pipetted into the 10^-4 tube (again to the 10^-6 and finally from that tube to the 10^-8 tube). After the dilutions were completed, 100 microliters from the 10^-2 plate was pipetted on the the 10^-3 nutrient agar plate and another 100 microliters onto the 10^-3 nutrient agar plate plus tetracycline plate. This was repeated for each tube and agar plate, the 10^-4 dilution on the 10^-5 plates ,the 10^-6 on the 10^-7 plates, and the 10^-8 on the 10^-9 plates. The plates were then placed agar side up on a rack and left to incubate for one week.
Data and Observations After viewing the hay infusion culture, it was clear that some brownish moldy looking type substance was floating on the top of the water in the jar. The liquid mixture appeared to be completely brown. It also had a strong moldy smell. Below is a picture of what the hay infusion culture looked like. When looking at the slide that was taken from the top left of the culture, I found many moving organisms that appeared to possibly be peranema. There were up to ten in view at one time. the were clear in color with a little green internal coloring. The cell was elongated and slightly rounded. When viewed in the 40x magnifications, the organisms appeared to be slightly vibrating. I also saw a cluster of what looked like tiny green cells. The individual cells were round but formed an irregular shape when linked together. This may have been gonium. The sample taken from the bottom right of the jar also appeared to have life present when viewed under the microscope. First I saw an organism that appeared to me moving very quickly. It was clear with some green internal coloring and very circular. The next organism I observed was clear with light pink coloring inside. It was very motile and I think this may have been Blepharisma. I estimate that I saw at least 150 of these organisms. Last I saw what I believe to be gonium again. They were very small green clusters of cells. The motile organisms meet the needs of life because they are made of at least one cell, they needed the jar to remain open for air, and they also needed food to survive. If the hay infusion culture continued to "grow" for another two months, I predict that the smell would definitely become much more pungent. I also think that much more mold would be visible to the naked eye growing within the culture.
Conclusions and Future Directions I predict that there will be much more growth of bacteria in the nutrient agar plates than the plates with the tetracycline antibiotic. The least diluted agar plates will probably have the most visible bacteria colonies, while the most diluted plates will probably show much less growth. It is also possible that fungus may appear in the agar plates as well.
CK
Study of Biological Life in an Ecosystem January 28, 2015
Purpose In this study both biotic and abiotic components were observed in an ecosystem. This portion of the study includes analyzing the specific 20 x 20 meter transect, specifically looking at what makes up this ecosystem. After a collection of the biotic and abiotic components was taken, a hay infusion culture was created to later observe protists, bacteria, and changes in the culture overtime.
Materials and Method After being assigned a specific transect to observe, my lab partners and I first drew a map of the area. Everything was labeled that was visible, for example, plants, soil, rocks, leaves, and even manmade objects. A list of five biotic and five abiotic components found in the transect was also composed. Then a sample consisting of soil and plants was put into a 50mL conical tube. After returning back to the lab, 11.7 grams of the sample was placed into a jar with 500mLs of water. Then 0.1 gm of dried milk was also added to the sample and mixed for about 10 seconds. The jar, known as a hay infusion culture, was then labeled with our transect number (5), and left in the lab for a week with the lid off.
Data and Observations Attached below is a hand drawn map of the transect as well as other pictures of the area. The 20x20 meter transect consisted of a larger grass area. It also contained an area of soil with bushes, weeds, berries, dead leaves, and twigs. Coming into one corner of our transect was also a man made concrete sign. 5 biotic components of the transect -bushes (possible rose plants) -grass clumps under bushes -grass -berries -weeds 5 abiotic components of the transect -soil -dead leaves -snow -manmade stone structure -wood chips/twigs
Conclusion and Future Directions It can be concluded that both biotic and abiotic components make up the transect. I predict that next week when observing the hay infusion culture, it will probably appear moldy and have a noticeable smell. When observing slides with samples taken from the culture, I expect to see protists within the sample.
http://openwetware.org/images/2/28/Map_for_bio.jpeg
CK
January 21, 2015
This is my first wetware post
CK