User:Esha B. Dholia/Notebook/Biology 210 at AU: Difference between revisions
| (69 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== '''Embryology & Zebrafish Development - February 18, 2015''' == | |||
[[Purpose:]] <p> The purpose of the examination of the Zebrafish is to learn and compare the stages of embryonic development in different organisms, and to set up an experiment to study how the environment can affect embryonic development. </p> | |||
[[Materials & Methods:]] <p> Before beginning any experimentation, each lab group was responsible for observing the development of frogs, starfish, chicks, and humans to note differences and similarities in how they developed, as well as the formation of different organisms in the organisms' embryology. Upon reading a paper about the effect of a certain external factor - caffeine, fluoride, salinity, etc. - on the development of the fish, students created a hypothesis, prediction, and experimental plan on how they themselves plan to test the effect of one such variable on zebrafish behavior. The embryos were observed and their developmental stage was determined. Then, a control group was set up, using 20 mL of Deerpark water. This lab group sought to test the effect of fluoride on Zebrafish development, and so two more groups were set up, one with a 10 mL Deerpark water - 10 mL fluoride ratio (the fluoride solution was of a 10 mg/L concentration), and a second completely of fluoride. Each plate was inoculated with 20 healthy, translucent embryos. Over the course of 2 weeks, the group was responsible for the observation of the fish and recorded qualitative data on their physical appearances and outward characteristics, as well as the collection of quantitative data (fish length, the number per colony that passed away, etc). </p> | |||
[[Results:]] <p> Table 1. The following table contains information about the progression of the three different plates of Zebrafish and their various physical characteristics. The full dose refers to the plate entirely of 20 mL fluoride; the half dose to 10 mL water and 10 mL fluoride solution; and the control 20 mL water. </p> | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Days Past''' | |||
| align="center" style="background:#f0f0f0;"|'''Type''' | |||
| align="center" style="background:#f0f0f0;"|'''# of Dead Organisms''' | |||
| align="center" style="background:#f0f0f0;"|'''# of Living Egg Cases''' | |||
| align="center" style="background:#f0f0f0;"|'''# of Larvae''' | |||
| align="center" style="background:#f0f0f0;"|'''Stage''' | |||
| align="center" style="background:#f0f0f0;"|'''Length (mm)''' | |||
| align="center" style="background:#f0f0f0;"|'''Description''' | |||
|- | |||
| 0||Control||0||20||0||Most were in 8 or 16 cell stages, two were in 60 hr stage||~ 1mm||Embryo is encased; rounded shapes at the top of the egg cases indicate the stages | |||
|- | |||
| 0||1/2 Dose||0||20||0||Either in 8 or 16 cell stage.||~ 1mm||Embryo is encased; rounded shapes at the top of the egg cases indicate the stages | |||
|- | |||
| 0||Full Dose||0||20||0||Either in 8 or 16 cell stage.||~ 1mm||Embryo is encased; rounded shapes at the top of the egg cases indicate the stages | |||
|- | |||
| 1||Control||0||20||0||All in 72 hr stage.||~ 2 mm||Eyes have behun to develop, retina pigmentation is relatively dark. | |||
|- | |||
| 1||1/2 Dose||1||19||0||All in 72 hr stage.||~ 2 mm||Eyes have behun to develop, retina pigmentation is relatively dark. | |||
|- | |||
| 1||Full Dose||2||18||0||All in 72 hr stage.||~ 2 mm||Eyes have behun to develop, retina pigmentation is relatively dark. | |||
|- | |||
| 4||Control||5||1||14||Most in early larvae stage.||~ 4 mm||Longer tails, mobile, mouths are protruding/more noticeable, pectoral fins are developing. | |||
|- | |||
| 4||1/2 Dose||0||1||18||Early larvae stage.||~ 3 mm||Longer tails, fin developing, mouth more noticeable, some are deformed with more curved shape | |||
|- | |||
| 4||Full Dose||3||0||15||Early larvae stage.||~ 3 mm||Deformities are showing (curved body instead of straight), but otherwise similr to 1/2 dose | |||
|- | |||
| 7||Control||9||0||5||72 hour stage||~1 mm||Long tail, dark eye pigmentation, fins have developed for the most part, eyes are moving, little fins are developing and moving near the fish's head, yolk absorbed, slightly protruding mouth, moving back and fourth (using fins to move), dark tail pigmentation, tail is thick | |||
|- | |||
| 7||½ Dose||4||0||14||72 hour stage||~1 mm||Skinnier tail than control; very dark eye pigmentation, fin has developed for the most part, slightly protruding jaw, more fidgety (keeps running into side of plate) swimming side to side using tail & fin, no yolk, no eye movement, slight tail pigmentation. | |||
|- | |||
| 7||Full Dose||8||0||7||72 hour stage||~1 mm||Mobility is very spastic (using fins and tails to move side to side, but not moving very far); end of tail is propelling fish forward; limited mobility; light pigmentation of tail; no eye movement and eyes seem significantly smaller & eyes are completely black; no yolk and pectoral fins are really skinny; flat jaw (not protruding). | |||
|- | |||
| 11||Control||5||0||0||> 72 hour stage||~2 mm||All dead; string-like pieces floating around, mold at center of plate, particles floating around plate. | |||
|- | |||
| 11||½ Dose||14||0||0||> 72 hour stage||~2 mm||None alive, there are string-like particles floating around the plate & molds growing. | |||
|- | |||
| 11||Full Dose||7||0||0||> 72 hour stage.||~2 mm||All dead; string-like pieces and moldy caracases/particles floating around the plate. | |||
|} | |||
[[Image:IMG 5311.JPG]] | |||
<p> Above is an image of the Zebrafish on Day 1. </p> | |||
[[Image:Day4fish.jpg]] | |||
<p> Above is an image of the Zebrafish on Day 4. </p> | |||
[[Image:Image2.jpeg]] | |||
<p> Above is an image of the control Zebrafish on Day 7. </p> | |||
[[Image:eyepigmentation123.jpg]] | |||
<p> Above is an image of the Zebrafish treated with the full dose on Day 7. Note the eye, body, and tail pigmentation.</p> | |||
[[Image:Image4.jpeg]] | |||
<p> Above is an image of the curved tail of the Zebrafish in the half dose on Day 7. </p> | |||
[[Image:Image8.jpeg]] | |||
<p> One of the mold-like substances found in stead of Zebrafish on Day 11 on the full dose plate. </p> | |||
<p> One of the Zebrafish from Day 7 was preserved in formaldehyde. It appears as follows: </p> | |||
[[Image:Image13.jpeg]] | |||
[[Conclusion:]] <p> It is hypothesized that higher concentrations of fluoride will negatively effect the Zebrafish, as demonstrated by what was written in the paper. This hypothesis was found to be partially correct. While the presence of fluoride certainly negatively affected the Zebrafish in the half dose and full dose plates negatively, in the end they all died around the same day. This death came earlier than expected in all of the fish, and is perhaps due to the fluoride but could also simply have had to do with evaporation of water in all the plates or some sort of nutritional deficit in the fish. In general, though, some of the adverse effects described in the paper - a decrease in length and decreased mobility - were certainly true in those fish treated with the fluoride, and these defects were more present in the fish in the full dose solutions versus the half dose solutions. </p> | |||
---- | |||
'''2.20.15''' | |||
Excellent lab book entry. | |||
Good description of invertebrate analysis and data. | |||
Good food web, a little small, hard to read. | |||
Well organized, I particularly like the contents section at the top of your notebook. | |||
'''SK''' | |||
=='''16S PCR Sequencing - February 26, 2015'''== | |||
[[Purpose:]] <p> The purpose of the PCR sequencing was to amplify the 16S rRNA gene found on bacteria. This sequence is unique to each separate bacteria, which makes it possible to distinguish between different organisms and identify those organisms in the transect. </p> | |||
[[Materials & Methods:]] <p> Upon identifying the bacteria found in the Hay Infusion Culture via the agar plates, a polymerase chain reaction (PCR) was set up. One colony from each the tetracycline plate and plain agar plate was chosen for two separate PCR amplifications. Each colony was placed into 100 microliters of water in a sterile tube and was denatured for 10 minutes on a 100 degrees C heat block. The samples were centrifuged at 13,400 rotations per minute for 5 minutes. During this, 20 microliters of a primer/water mixture was added to a PCR tube and then mixed with a PCR bead. Five microliters of the supernatant from the centrifuged sample was transferred to the tube and then placed in a PCR machine. After one week, the PCR products were run through an agarose gel. </p> | |||
[[Data & Observations:]] <p> Because the PCR sequence this lab group turned out to have a purified product, a DNA sequence was found for each of the two bacteria, as follows: </p> | |||
<p> MB47-For_16S_G06.ab1 NNNNNNNNNNNNNNNNNCTNNNCNTGCAGCCGAGCGGTAGAGATTCTTCGGAATCTTGAGAGCGGCGCACGGGTGCGGAA CACGTGTGCAACCTGCCTTTATCAGGGGAATAGCCTTTCGAAAGGAAGATTAATGCCCCATAATATATCATATGGCATCA TTTGATATTGAAAACTCCGGTGGATAAAGATGGGCACGCGCAGGATTAGATAGTTGGTAGGGTAACGGCCTACCAAGTCA GCGATCCTTAGGGGGCCTGAGAGGGTGATCCCCCACACTGGTACTGAGACACGGACCAGACTCCTACGGGAGGCAGCAGT GAGGAATATTGGACAATGGGTGAGAGCCTGATCCAGCCATCCCGCGTGAAGGACGACGGCCCTATGGGTTGTAAACTTCT TTTGTATAGGGATAAACCTACCCTCGTGAGGGTAGCTGAAGGTACTATACGAATAAGCACCGGCTAACTCCGTGCCAGCA GCCGCGGTAATACGGAGGGTGCAAGCGTTATCCGGATTTATTGGGTTTAAAGGGTCCGTANGCTGATGTGTAANTCANTG GTGAAATCTCACANCTTANCTGTGAAACTGCCNTTGATACTGCATGTCTTGAGTGTTGTTGAANTANCTGGAATAANTNN GTANCAGTGAAATGCCTANATATTACTTNNANCACNANGTGCTAANGCANGTTGGTANNCCNCNACTGACNCTGATNGAG NAAANCNTGGGNNAGCGAACANAANTNNATACCCTGGGGNNGTNNNCNNNAANNAANCTNANTCNNTTTTTNTCTTTCTC TTNCNNATACANNNNNANCCGANAAGNTNGCCNNCTNCCGGGTGGTGTTCTCCNTNNTNNNGATGNNNTCNNCTNNNNNN NNNNNCNGCCCCCCCNCAANNATTTNTANANNNNTATANNNTNNNANANCNNGCGGCCCCCTNTNTAANNGGNNNNGGGG GAGNNNNNNGNNNNNGTTTTCTATTATATNTNNNNCTNTNNNCCNCNNGNNCNGGGGGGGTTGTNTCTCCCNNCCAGAAC NNAANGANANTNTNCNNCANCAGCCNNNN </p> | |||
<p> MB48-For_16S_H06.ab1 NNNNNNNNNNNNNNGCNNANNNTGNNANNNNNGCGGTANGANGGGANGCTTGCTCTNNGATTCAGCGGCGGACGGGTGAG TAATGCCTAGGAATCTGCCTGGTAGTGGGGGACAACGTTTCGAAAGGAACGCTAATACCGCATACGTCCTACGGGAGAAA GCAGGGGACCTTCGGGCCTTGCGCTATCAGATGAGCCTAGGTCGGATTAGCTAGTTGGTGAGGTAATGGCTCACCAAGGC GACGATCCGTAACTGGTCTGAGAGGATGATCAGTCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAG TGGGGAATATTGGACAATGGGCGAAAGCCTGATCCAGCCATGCCGCGTGTGTGAAGAAGGTCTTCGGATTGTAAAGCACT TTAAGTTGGGAGGAAGGGCATTAACCTAATACCTTGGTGTTTTGACGTTACCGACAGAATAAGCACCGGCTAACTCTGTG CCAGCAGCCGCGGTAATACAGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCGCGTANGTGGTTTGTTAAG TTGGATGTGAAAGCCCCGGGCTCAACCTGGGAACTGCNTTCNAAACTGNCNAGCTAGAGTATGGTANAGGGTGGTGGAAT TTCCTGTGTAGCNNTGAAATGCGTAGATATANGAANGAACACNNNGTGGCNAAGCGGACCACCTGNACTGATACTNACAC TGANNTGCGAAANNNTGTGGANCAAACANNATTANGATNNCCTNNAGTCCACNGCCNGTANACNNNNTCAACTANCNNNN NNAGCNCTTNANNTGTTANTGNCGCNNCTAACNCATTAANTNNCNNCGCTGGNTNNGTAGNAGNCCNCGNCCGTTAGNNC TNNNNNNGGAGTTNANNGGNGCCNNGCACAAGCNACTGNAGCAGGGNGGGNTGTAGTTCCNAANNNNNNACNAAAAANNN NNACCCNGNCCCTNGGNATNNAANNNAGNNNNNGAGGNNNNNNAANNNGNGNNNNGGNTGNNNNCNNNGGAAANNNNACC ANNNGNNNATGGTNGGNNNNNNNCNNCANNNNNNCNANCCCNNNNNNN </p> | |||
<p>[[Conclusion:]] Using an online website, the lab group was able to determine the species of the two bacteria. The first sequence pointed to the bacteria being a Chryseobacterium, and this is consistent with the observations made in the lab; the chryseobacteria was gram negative, circular, and moves in a gliding motion. These bacteria can be found in many different organisms and have many varieties, including raw milk. The bacteria of the second sequence matched the Pseudomonas aeruginosa. This also matches the observations made; the bacteria is gram negative, coccus or bacillus in shape, and has some motility. </p> | |||
== '''Invertebrates - February 12, 2015''' == | |||
[[Purpose:]] | |||
<p> The purpose of this lab is to understand the importance of invertebrates and to understand the evolution of simple systems into more complex ones, which is how the vertebrate organ system developed from these simple organisms. </p> | |||
[[Materials & Methods:]] | |||
<p> ''Procedure 1:'' Each lab group began by observing three different types of worms - the acoelomate Planaria (a flatworm), a nematode (a roundworm), and the coelomate Annelida (an earthworm) - under microscopes. Observing these three invertebrates was meant to demonstrate how the worms' different forms of movement related to their body structure. </p> | |||
<p> ''Procedure 2:'' In this portion of the lab, students were to observe example invertebrates from the five major classes: arachnida, diplopoda, chilopoda, insect, and crustacea. Students were meant to differentiate between the organisms based on their body parts and the number of appendages they each had. </p> | |||
<p> ''Procedure 3:'' In this portion of the lab, students were sent to observe the invertebrates found in the Berlese Funnel. After breaking down the funnel, each lab group poured the top 10-15 mL of liquid and organisms into a petri dish, and the remaining liquid into another. Then, using a dissecting microscope, each lab group attempted to identify the class of Arthropoda invertebrates using a key. Observations were recorded in a table. </p> | |||
<p> ''Procedure 4:'' This final part of the lab - and a culmination of the study - had students consider the vertebrates that pass through the transect, whether those vertebrates be a niche for the organisms or simply part of the niche. Students identified five of these organisms, determined their classification, and considered the biotic and abiotic characteristics of their particular transects that would benefit each species. Then, a food web was created based on every organism observed in the transect. </p> | |||
[[Data & Observations:]] | |||
<p> All three of the worms moved by contracting and releasing their muscles. Though evolutionarily they might appear to be different, their movement in this regard seems to be similar. </p> | |||
<p> The table below provides a detailed description of the five invertebrates found in the Berlese Funnel: </p> | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Organism''' | |||
| align="center" style="background:#f0f0f0;"|'''Length (mm)''' | |||
| align="center" style="background:#f0f0f0;"|'''# in Sample''' | |||
| align="center" style="background:#f0f0f0;"|'''Description of Organism''' | |||
|- | |||
| Springtail (Phylum: Athropoda; Class: Hexopoda) ||Appx. 10 mm||2||Antannea preseent; hairlike features; 3-6 legs; segmented body | |||
|- | |||
| Soil Mite (Phylum: Arthropoda; Class: Arachnida) ||Appx. 1 mm ||20||Very small, circular, non-segmented body, many present | |||
|- | |||
| Pseudoscorpions (Phylum: Athropoda; Class: Arachnida) ||Appx. 8 mm||4||Very small, segmented body, two piercers preesent | |||
|- | |||
| Beetle Larvae (Phylum: Arthropod; Class: Insecta) ||Appx. 5 mm||1||Small, circular in shape, few legs/hairlike features | |||
|- | |||
| Scale or Aphid (Phylum: Arthropoda; Class: Insecta) ||Appx. 20 mm||1||Large in size, striped, harilike features, 6 legs, long antaneea | |||
|} | |||
<p> ''The following are photos of the organisms found in the transect:'' </p> | |||
[[Image:Springtail.jpg]] | |||
<p> In the image above, the large organism is the Springtail. Above it is a Soil Mite. </p> | |||
[[Image:Pseudoscorpion.jpg]] | |||
<p> In the image above, a Pseudoscorpion is shown to the left, and on the right is a second Soil Mite. </p> | |||
[[Image:Beetle larva.jpg]] | |||
<p> Pictured above are two beetle larvae. </p> | |||
[[Image:Scale or aphid.jpg]] | |||
<p> Pictured above is a scale or aphid. </p> | |||
<p> Below is a chart that classifies and describes the various vertebrates that are predicted to live in or pass through the transect. Abiotic and biotic factors of the transect that the organisms could find beneficial are listed below. </p> | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Vertebrate''' | |||
| align="center" style="background:#f0f0f0;"|'''Classification (Phylum/Genus/Species)''' | |||
| align="center" style="background:#f0f0f0;"|'''Beneficial Biotic''' | |||
| align="center" style="background:#f0f0f0;"|'''Beneficial Abiotic''' | |||
|- | |||
| Chipmunk ||Chordate/Tamias/T. alpinus||Lots of seeds of plants in the planters to be eaten||Chipmunks can burrow into the soil | |||
|- | |||
| Deer||Chordata/Odocoileus/O. virginianus||Lots of vegetation to consume||Low fences/planters are easy to climb over, mulch is not difficult to tread on | |||
|- | |||
| Hawk||Chordata/Buteo/B. jamaicicenis||Can eat organisms such as rats that frequent the transect||Dead weeds at the top of transect can be used to build nest, as can branches/branch-like pieces of mulch | |||
|- | |||
| Rat||Chordata/Rattus/R. norvegicus||Can burrow into the plants for shelter & consume them if hungry||Can burrow into the soil/under the planters for safety | |||
|- | |||
| Robin||Chordata/Turdus/T. migratorius||Lots of seeds of plants in the planters to be eaten||Dead weeds at the top of transect can be used to build nest, as can branches/branch-like pieces of mulch | |||
|} | |||
<p> Below is a food web of all the organisms - vertebrates, invertebrates, and plants - found in the transect. The food web describes the passage of energy from each level of organism to the next. </p> | |||
[[Image:Eshafoodweb.jpg]] | |||
[[Results:]] | |||
The observing of and classification of invertebrates found in the Berlese funnel through this experiment led to a greater understanding of the biodiversity found in such a small area of land. The analysis of the vertebrates provided this same understanding. Creation of the food web allowed a look at this particular community, or all the intersections of different species in this one particular geographical area. The leveling of the food chain allowed for the separation of organisms into their separate trophic levels, or the position an organism occupies in a good chain. The producers - organisms that make their own food - were found at the bottom, and following them were primary, secondary, and tertiary consumers. The energy being transferred from each trophic level decreases as the levels increase, which could account for why most food webs appear to have more producers than tertiary consumers. It is unknown whether or not this particular transect has reached its carrying capacity, or the maximum number of organisms that can live in the area. | |||
*'''[[User:Esha B. Dholia|Esha B. Dholia]] 02:19, 18 February 2015 (EST)''': | |||
---- | |||
'''2.20.15''' | |||
Excellent notebook entry. | |||
'''SK''' | |||
== '''Plantae & Fungi - February 5, 2015''' == | |||
[[Purpose:]] The objectives of this lab were to understand the various characteristics and diversity of plants, and to appreciate the function and role fungi play in everyday life. | |||
[[Materials & Methods:]] | |||
<p> ''Procedure 1:'' Each lab group obtained two to three Ziploc bags and proceeded to their transects. In the first bag, each group was required to collect a leaf litter sample, which would be composed of about 500g of dead leaves and plant matter. The bag should have contained about 80% leaves and only 20% of the crumbly soil. The second bag should have contianed a representative sample from five different plants in the transect in a way that would be minimally damaging. This included collecting any seeds, pine cones, and flowers in the transect, and would be used in the lab to identify the major group and genus of each of the five samples. </p> | |||
<p> ''Procedure 2:'' In order to understand what genus the plant samples are from, each lab group observed different characteristics of the plant, including plant vascularization. Each group first observed a moss and a lily plant stem. They then observed a cross section of the lily stem, looking specifically for xylem and phloem layers. Based on these observations, each lab group then was to characterize the vascularization in each of the plants from their transects. </p> | |||
<p> ''Procedure 3:'' Another characteristic the lab groups observed was the presence of specialized structures in the plants. The lab groups were to describe the shape, size, and cluster arrangement of the leave from their transect plants, as well as the attachment sites of leaves on these plants and evidence of leaves in the area via leaf litter. </p> | |||
<p> ''Procedure 4:'' A third characteristic the lab groups observed was the mechanism of plant reproduction. The goal of this portion of the lab was to determine the way in which the plants in the transect reproduce. After observing moss, a lily flower, and seeds, each lab group was to observe seeds collected from their transects to identify if they were monocot or dicot, and whether or not there was evidence of flowering or spores. In this particular lab group, which was responsible for transect 4, no seeds were found for fear of disrupting the environment. </p> | |||
<p> ''Procedure 5:'' This portion of the lab called for observing fungi and identifying the different structures and characteristics that make fungi important. Each lab group was responsible for looking at some of the samples and deciding whether or not the organisms were fungi, and which of the three groups they belonged to. </p> | |||
<p> ''Procedure 6:'' The final portion of this lab was setting up a Berlese Funnel to collect invertebrates from the transect. The leaf litter Ziploc bag was to be used in this portion of the lab. Each lab group poured 25 mL of a 50/50 ethanol-water solution into a 50 mL conical tube. After fitting a piece of screening material into the bottom of the funnel, the leaf littler sample was carefully put in the top of the funnel. The funnel was then set up on a ring stand so it would be held in the tube with the ethanol, and then the base of the funnel was parafilmed so the ethanol would not evaporate. A lighted watt lamp was placed above the funnel with an incandescent lightbulb about 1-2 inches from the top of leaf litter, and everything was covered with foil. The Berlese funnel was left on the lab bench for one week for the invertebrates to grow. </p> | |||
[[Data & Observations:]] | |||
<p> The following table contains information about the five plants collected from Transect 4, including each plant's method of vascularization, reproduction, specialized structure, shape, and size. </p> | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Transect Sample Plants''' | |||
| align="center" style="background:#f0f0f0;"|'''Location & # in Transect''' | |||
| align="center" style="background:#f0f0f0;"|'''Description (Size & Shape)''' | |||
| align="center" style="background:#f0f0f0;"|'''Vascularization''' | |||
| align="center" style="background:#f0f0f0;"|'''Specialized Structures''' | |||
| align="center" style="background:#f0f0f0;"|'''Mechanisms of Reproduction''' | |||
|- | |||
| 1||Third planter box, brussel sprouts||Brussels sprout leaf, very large (19 cm from stem to top, veins are crisscrossed, thick stem, leaf has waxy, wavy, hard texture)||Xylem & phloem||Chlorophyll, ovary, & ovules||Alternation of generations | |||
|- | |||
| 2||First planter box, kale||Thick stem, crisscrossed veins, curly/wavy leaves, hard in texture, large sized leaves, 11 cm from root to tip||Xylem & phloem||Guard cells, stomata, chlorophyll||Alternation of generations | |||
|- | |||
| 3||Fourth planter box, lettuce||Small stem, flat veins are parallel to one another, soft texture, smaller leaves, 9.5 cm from top of leaf to bottom||Xylem & phloem||Guard cells, stomata, chlorophyll||Alternation of generations | |||
|- | |||
| 4||Second planter box, grass||Short, similar-sized roots, one part red & other part green, no visible veins||Xylem & phloem||Chlorophyll, stomata, guard cells||Alternation of generations | |||
|- | |||
| 5||Fourth planter box, weed||Weed, many leaves, crisscrossed veins, small in size (about 2.4 cm sized leaves), small ridges and rounded leaves||Xylem & phloem||Chlorophyll, stomata, guard cells||Alternation of generations | |||
|} | |||
<p> The following are photos of the five different samples collected from Transect 4. </p> | |||
[[Image:Lab 4 brussel lead.jpeg]] | |||
<p> Sample one is the brussels sprout leaf collected from one of the planters. </p> | |||
[[Image:Esha kale.jpeg]] | |||
<p> Sample two is one of the kale leaves collected from one of the planters. </p> | |||
[[Image:Esha lettucce.jpeg]] | |||
<p> Sample three is a lettuce leaf collected from one of the planters. </p> | |||
[[Image:Weed esha.jpeg]] | |||
<p> Sample five is a weed collected from one of the planters. </p> | |||
The specimen observed in Procedure 5 are classified in the table below: | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''Bread Mold''' | |||
| align="center" style="background:#f0f0f0;"|'''Rhizopus Nigricans''' | |||
| align="center" style="background:#f0f0f0;"|'''Mushroom''' | |||
|- | |||
| Fungi?||Yes||Yes||Yes | |||
|- | |||
| Classification||Ascomycata||Zygomycata||Basidiomycota | |||
|} | |||
<p> The following is an image of Rhizopus stolonifer, which was found to be a zygomycata. It can be classified as a fungi because it has many sporangia, which in fungi are the site of meiosis and asexual reproduction (http://www.britannica.com/EBchecked/topic/222357/fungus/57965/Sporophores-and-spores#ref519195). </p> | |||
[[Image:Fungii.jpeg]] | |||
[[Conclusion:]] | |||
<p> Observing the various plants in Transect 4 allowed for a greater understanding of the immense diversity of plants. Most of the plants in Transect 4 appeared to have similar methods of reproduction, specialized structures, and methods of vascularization, which makes sense seeing as they are all garden plants and being grown in similar environments, which accounts for their many similarities. Future observation of the soil and leaf litter left for development in the Burlese funnel will be interesting and assist in making greater sense of the transect and its organisms. </p> | |||
<p> --[[User:Esha B. Dholia|Esha B. Dholia]] 21:20, 11 February 2015 (EST) </p> | |||
<p>'''2.10.15''' Excellent notebook entry. Plenty of detail in methods and observations. Nice images and good formatting.''' SK'''</p> | |||
== '''Observing Antibiotic Resistance & Identifying Bacteria with DNA Sequences - January 29, 2015 ''' == | |||
[[Purpose:]] | |||
<p> The purpose of this lab was to understand the different characteristics of bacteria, observe and understand antibiotic resistance in these bacteria, and understand how DNA sequences can be used to identify different species. In the previous lab, eight agar plates, four with the antibiotic tetracycline, were plated with various dilutions of the bacteria cultures for each group's transect. It is hypothesized bacteria will have grown on the plates, but not archaea, because archaea only tend to grown in extreme environments and these plates were simply left at room temperature for a week. It is also hypothesized that the hay culture will have changed in appearance sine last week. This change in appearance can be attributed to the change in time; as the weeks have progressed, bacteria and other living organisms have had time to grow and change in appearance. </p> | |||
[[Materials & Methods:]] | |||
<p> ''Procedure 1:'' Each lab group retrieved their eight agar plates inoculated from the Hay Infusion Culture to count the total number of colonies per plate. Depending on the plate and whether or not tetracycline was present or not, each lab group could expect to see a variety of different numbers of cultures on their plates. </p> | |||
<p> ''Procedure 2:'' Each lab group then considered the differences in the number of colonies on plates with and without the antibiotic, and attempted to determine the mechanisms which could have caused resistance in the bacteria to the tetracycline. </p> | |||
<p> ''Procedure 3:'' Each lab group was to observe four different samples of microorganisms, two from the nutrient agar plates and two from the nutrient agar plus tetracycline plates. For each sample, a wet mount and a gram stain was to be prepared; then, the samples were to be observed using a high-power lens or the 100x oil immersion technique. To prepare the wet mount, a loop was sterilized over a flame with alcohol and used to scrape up one of the growths off the surface of one of the agar plates. This small colony was then mixed with a drop of water on a slide, covered with a cover slip, and observed under the microscope. Because it was difficult to see the organisms because they were not stained, the oil immersion at 100x was an option to see the bacteria better. The cell shapes and motility of the organisms were determined via the wet mount. To prepare a gram stain, the loop was sterilized over a flame and used to scrape up a small amount of the growth from the agar. It was mixed with a drop of water on a slide. The area beneath the sample was circled with a red wax pencil, and the slide was labeled with the name of the agar plate. Then, the slide and its contents were heat fixed by passing the slide through a flame three times with the bacterial smear side up. In a staining tray, the bacterial smear was covered with crystal violet for one minute, and then rinsed off using a wash bottle filled with water. The smear was then covered with Gram's iodine mordant for one minute, and again washed with water. To decolorize the bacterial smear, a rinse with 95% alcohol was applied to the slide for 10-20 seconds. The smear was then covered with a safranin stain for 20-30 seconds and rinsed with water. The excess water was blotted away with a kimwipe and the slide was allowed to air dry. Then, the gram stained sample was observed under the microscope. The gram stain procedure and the wet mount procedures were used for all four samples. </p> | |||
<p> ''Procedure 4:'' Each group characterized four bacteria from the Hay Infusion Culture, two from each of the two types of plate. One bacteria from each of the two plates was selected for PCR analysis to amplify the 16S rRNA gene, a sequence that is specific to each species, which will occur in the next lab. The colonies were each transferred to 100 microliters of water in two different sterile tubes. They were incubated at 100 degrees Celsius for 10 minutes in a heat block, and then centrifuged for 5 minutes at 13400 rpm. During the centrifugation, 20 microliters of primer were added to a labeled PCR tube, and mixed to dissolve the PCR bead. Finally, 5 microliters of the supernatant from the centrifuged sample were transferred to the 16S PCR reaction. The tubes were then placed in the PCR machine. </p> | |||
[[Data & Observations:]] Before carrying out Procedure 1, it was noted that the Hay Infusion Culture did not have any scent, but was very brown and the film that had persisted on the sides of the culture's container was present and perhaps thicker than it had previously been. The following table contain the results of the 100-fold serial dilutions results: | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Dilution''' | |||
| align="center" style="background:#f0f0f0;"|'''Agar Type''' | |||
| align="center" style="background:#f0f0f0;"|'''Colonies Counted''' | |||
| align="center" style="background:#f0f0f0;"|'''Conversion Factor''' | |||
| align="center" style="background:#f0f0f0;"|'''Colonies/mL''' | |||
|- | |||
| 10^-3||Nutrient||37||x 10^3||37000 | |||
|- | |||
| 10^-5||Nutrient||17||x 10^5||1700000 | |||
|- | |||
| 10^-7||Nutrient||1||x 10^7||10000000 | |||
|- | |||
| 10^-9||Nutrient||0||x 10^9||0 | |||
|- | |||
| 10^-3||Nutrient + tet||14||x 10^3||14000 | |||
|- | |||
| 10^-5||Nutrient + tet||0||x 10^5||0 | |||
|- | |||
| 10^-7||Nutrient + tet||0||x 10^7||0 | |||
|- | |||
| 10^-9||Nutrient + tet||0||x 10^9||0 | |||
|} | |||
For the morphology observations, the 10^-3 plates with and without tetracycline were chosen. A yellow culture and a white culture were chosen from each of these plates to represent bacterial diversity. The following are photographs of the bacteria without the gram-staining: | |||
[[Image:10^-3 .jpeg]] | |||
<p> The 10^-3 culture, before any tests were ran. </p> | |||
[[Image:10^-3 tet.jpeg]] | |||
<p> The 10^-3 culture plus tetracycline, before any tests were ran. </p> | |||
[[Image:White 10^-3 unstained.jpeg]] | |||
<p> A white colony from the 10^-3 plate was sampled and observed under a microscope without any staining at 40x. They are very small and difficult to see. </p> | |||
[[Image:White t10^-3 unstained.jpeg]] | |||
<p> A white colony from the 10^-3 plate with tetracycline was sampled and observed under a microscope without any staining. The string-like arrangement of the cells is apparent here. </p> | |||
[[Image:Yellow 10^-3 unstained.jpeg]] | |||
<p> A yellow colony from the 10^-3 plate was sampled and observed under a microscope without any staining. The cells appear raised and in string-like arrangements, and have rod shapes. </p> | |||
The following are photographs of the bacteria after the gram-staining: | |||
[[Image:White 10^-3 stained.jpeg]] | |||
<p> The white 10^-3 colony after staining. The violet color indicates peptidoglycan is present in the cell walls. </p> | |||
[[Image:White t10^-3 stained.jpeg]] | |||
<p> The white 10^-3 colony with tetracycline after staining. Again, the violet color indicates peptidoglycan is present in the cell walls. </p> | |||
[[Image:Yellow 10^-3 stained.jpeg]] | |||
<p> The yellow 10^-3 colony after staining. Because the bacteria appear pink, it can be inferred that they are gram-negative and do not have peptidoglycan. </p> | |||
[[Image:Yellow t10^-3 stained.jpeg]] | |||
<p> The yellow 10^-3 colony after staining. There are not many bacteria present in this view; however, because they are violet, it is known that peptidoglycan is present in their cell walls. </p> | |||
The following table synthesizes all the information about the morphologies of the bacterial colonies and cells observed, both before and after staining. | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Colony Label''' | |||
| align="center" style="background:#f0f0f0;"|'''Plate Type''' | |||
| align="center" style="background:#f0f0f0;"|'''Colony Description''' | |||
| align="center" style="background:#f0f0f0;"|'''Cell Description''' | |||
| align="center" style="background:#f0f0f0;"|'''Gram +/-''' | |||
|- | |||
| White||10^-3||Approximately. .5 cm across, small, white. Entire & undulated edge. Flat elevation. Glistening, smooth surface. Circular.||Cells are vibrating, circular, and very close together. About 5 micrometers at 40x.||+ | |||
|- | |||
| Yellow||10^-3||Colony is circular, smooth and shiny. Irregular shape, filamentous edges, and wrinkled surface. Flat elevation, approximately. .5 cm across.||Cells are rod-shaped and arranged in long strings. They appear to look like raised palisades. Cells are not motile, though this could have been due to lack of water ion the slide. About 1 micrometer at 100x.||+ | |||
|- | |||
| White||T10^-3||Circular colony shape. Edges are entire, surface is smooth and glistening, elevation is convex. Small, approximately .2 cm in diameter.||Cells are arranged in string-like patterns and are mainly circular, though some are ovular. They bear resemblance to streptobacilli. About 5 micrometers at 40x.||- | |||
|- | |||
| Yellow||T10^-3||Circular colony shape. Edges are entire, surface is smooth and glistening, elevation is convex. Approximately .5 cm in diameter.||Coccus in shape, circular, individually dispersed through culture and not in groups, not motile. There are not many cells present on the slide. About 4 micrometers at 40x.||+ | |||
|} | |||
[[Conclusions & Future Directions:]] <p> After examining the colony types between the plates with and without the antibiotic, it was found that the plates with just the broth had more colonies than the plates with the broth and the tetracycline. This is because of bacterial resistance and natural selection. Only some of the bacteria on the tet plates were resistant to tetracycline; those that were not did not survive, but those that were survived to reproduce and form the colonies seen on the plates. There was one mold growing on the 10^-3 plate with tetracycline, but all the others were bacterial cultures. Bacteria that are resistant to tetracycline employ one of three mechanisms: tetracycline efflux, ribosome protection, and tetracycline modification (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC358256/). Each of these mechanisms has to do with the genotype of a particular bacteria, and is passed down from generation to generation genetically, allowing for natural selection and consequently evolution to do their work. Overall, tetracycline decreases the number of bacteria by weeding out the ones that are not resistant to the antibiotic. *'''ED''':</p> | |||
'''2.4.15''' Excellent notebook entry. Included lots of detailed description of Hay Infusion and location of protists observed. Good description of protists and identification. '''SK''' | |||
== '''Hay Infusion Culture Observations - January 22, 2015''' == | == '''Hay Infusion Culture Observations - January 22, 2015''' == | ||
| Line 7: | Line 331: | ||
<p> ''Procedure I - Using the Dichotomous Key:'' To become comfortable with the Dichotomous lab group was to make a wet mount of known organisms and observe them under the microscope at 40x objective. Then, a single organism had to be focused on and described by its size, shape, and various features. A Dichotomous key was then obtained and used to confirm the identity of the known organism by following the diagrams. </p> | <p> ''Procedure I - Using the Dichotomous Key:'' To become comfortable with the Dichotomous lab group was to make a wet mount of known organisms and observe them under the microscope at 40x objective. Then, a single organism had to be focused on and described by its size, shape, and various features. A Dichotomous key was then obtained and used to confirm the identity of the known organism by following the diagrams. </p> | ||
<p> ''Procedure II - Hay Infusion Observations:'' Each group brought the culture back to their work areas to note any scents and describe the cultures' appearances. Then, each group took two samples from different niches of the culture, making sure to include some plant matter, and prepared two different wet mounts. Then, observing the slides under the microscopes, each group used the Dichotomous key to determine which algae and protists were present. The group drew pictures of the organisms observed and recorded their size. Each group was responsible for finding at least 3 different organisms from each of the two niches. </p> | <p> ''Procedure II - Hay Infusion Observations:'' Each group brought the culture back to their work areas to note any scents and describe the cultures' appearances. Then, each group took two samples from different niches of the culture, making sure to include some plant matter, and prepared two different wet mounts. Then, observing the slides under the microscopes, each group used the Dichotomous key to determine which algae and protists were present. The group drew pictures of the organisms observed and recorded their size. Each group was responsible for finding at least 3 different organisms from each of the two niches. </p> | ||
<p>''Procedure III - Preparing & Plating Serial Dilutions:'' Upon completing the Hay Infusion, a serial dilution was to be plated, which would allow prokaryotic organisms and possibly fungi from the culture to be observed. To do this, 4 tubes of 10 mL sterile broth were labeled with 10^-2, 10^-4, 10^-6, and 10^-8. Four nutrient agar plates and four nutrient agar plus tetracycline plates were then obtained. One plate from each of the groups was labeled with 10^-3, 10^-5, 10^-7, and 10^-9, with the tetracycline plates labels prefaced with "tet." Then, using a micropipettor, 100 microliters of the culture was added to the broth in the 10^-2 tube | <p>''Procedure III - Preparing & Plating Serial Dilutions:'' Upon completing the Hay Infusion, a serial dilution was to be plated, which would allow prokaryotic organisms and possibly fungi from the culture to be observed. To do this, 4 tubes of 10 mL sterile broth were labeled with 10^-2, 10^-4, 10^-6, and 10^-8. Four nutrient agar plates and four nutrient agar plus tetracycline plates were then obtained. One plate from each of the groups was labeled with 10^-3, 10^-5, 10^-7, and 10^-9, with the tetracycline plates labels prefaced with "tet." Then, using a micropipettor, 100 microliters of the culture was added to the broth in the 10^-2 tube. The tube was then mixed thoroughly. One hundred microliters of broth from the 10^-2 tube was added to the 10^-4 tube, and swirled well. This was repeated to make the 10^-6 and 10^-8 dilutions. To plate these dilutions on the agar plates, 100 microliters from the 10^-2 tube was pipetted onto the plate labeled 10^-3. The sample was carefully spread on the plate, and the procedure was repeated on the 10^-3 plate containing tetracycline. This process was repeated to add the 10^-4 dilution to the 10^-5 plate, the 10^-6 dilution to the 10^-7 plate, and the 10^-8 dilution to the 10^-9 plate. The plates were placed on a rack to incubate at room temperature for one week. The process is depicted below.</p> | ||
[[Image:Esha2.jpg]] | |||
[[Data & Observations:]] | |||
Upon bringing the culture back to the table, it was noted that the culture had no scent and had taken on a brownish color. There was dirt floating in the culture at the bottom, and a thin brown film coated the top of the culture as well as the sides of the jar it was in, but there were no apparent signs of life. | |||
The first sample was taken from the top of the culture, where a cloudy film of dirt was noted. Three different organisms - chilomonas, colpidium, and paramecium aurelia - were observed. Each of the organisms appeared to be moving quickly and had some sort of mechanism to propel it along. The chilomonas and paramecium both had cilia all over their body, while the colpidium had two flagella. The three organisms were protozoa, because they did not have any green coloring and therefore could not have been photosynthetic organisms. The colpidium was oval-shaped and 51 micrometers, the chilomonas was 22 um, and the parameciumm was 156 um. The following is a picture of the site where the paramecium was seen: | |||
[[Image:Esha3.PNG]] | |||
The second sample taken came from the soil at the bottom of the culture. Here, a peranema, arcella, and paramecium bursaria were identified. The peranema and paramecium were both more motile than the arcella, which was creeping slowly around the sample. While the peranema and arcella were colorless and therefore could be presumed to be protists, the paramecium itself was green in color, meaning it is photosynthetic. This makes sense, as it was found close to the clump of dirt in which plants may have been growing. The arcella was 63 um, the paramecium was 156 um, and the paranema was 45 um. Pictured below is the sample of soil where the peranema was seen: | |||
[[Image:Esha4.JPG]] | |||
[[Conclusions:]] | |||
This lab demonstrated the diversity of organisms in niche, even though the niche was so small. Organisms differ where they are, if they're closet to plant matter or not. If organisms are closer to plant matter, it's likely they themselves are photosynthetic algae and produce their own food. As with the organisms in this niche, because much plant matter was not present, the observed organisms were all protists. The arcella, like all the organisms, satisfies all the needs of life: it has to acquire energy to survive and reproduce, which it does through consumption of nutrients externally. It's a unicellular organism and has its own genetic information, which allow it to stay alive. The arcella reproduces asexually by replicating its genetic information and then passing down identical versions of it to offspring, and overtime the arcella likely evolves, based on mutations in its genes and how adapted the protist is to its external environment. If the culture were to grow for another two months, it's possible plants would actually grown, seeing as the soil sample was taken from a garden, but it's also possible the soil would continue to sit and disintegrate at the bottom of the culture. Those communities within the culture that are reliant on the large chunks of soil at the bottom would be most affected by this and would no longer have the soil as a mechanism to cope. Selection would weed out those organisms that have low fitnesses and are not well-adapted to the environment. | |||
*'''Esha B. Dholia''': | |||
'''1.27.15''' | <p> '''1.27.15''' | ||
Good first entry. Missing some information eg. the Hay infusion set up. The transect was 20Ft by 20Ft. We will work on uploading pictures this week. | Good first entry. Missing some information eg. the Hay infusion set up. The transect was 20Ft by 20Ft. We will work on uploading pictures this week. | ||
SK | SK | ||
Latest revision as of 12:30, 24 March 2015
Embryology & Zebrafish Development - February 18, 2015
The purpose of the examination of the Zebrafish is to learn and compare the stages of embryonic development in different organisms, and to set up an experiment to study how the environment can affect embryonic development.
Before beginning any experimentation, each lab group was responsible for observing the development of frogs, starfish, chicks, and humans to note differences and similarities in how they developed, as well as the formation of different organisms in the organisms' embryology. Upon reading a paper about the effect of a certain external factor - caffeine, fluoride, salinity, etc. - on the development of the fish, students created a hypothesis, prediction, and experimental plan on how they themselves plan to test the effect of one such variable on zebrafish behavior. The embryos were observed and their developmental stage was determined. Then, a control group was set up, using 20 mL of Deerpark water. This lab group sought to test the effect of fluoride on Zebrafish development, and so two more groups were set up, one with a 10 mL Deerpark water - 10 mL fluoride ratio (the fluoride solution was of a 10 mg/L concentration), and a second completely of fluoride. Each plate was inoculated with 20 healthy, translucent embryos. Over the course of 2 weeks, the group was responsible for the observation of the fish and recorded qualitative data on their physical appearances and outward characteristics, as well as the collection of quantitative data (fish length, the number per colony that passed away, etc).
Table 1. The following table contains information about the progression of the three different plates of Zebrafish and their various physical characteristics. The full dose refers to the plate entirely of 20 mL fluoride; the half dose to 10 mL water and 10 mL fluoride solution; and the control 20 mL water.
| Days Past | Type | # of Dead Organisms | # of Living Egg Cases | # of Larvae | Stage | Length (mm) | Description |
| 0 | Control | 0 | 20 | 0 | Most were in 8 or 16 cell stages, two were in 60 hr stage | ~ 1mm | Embryo is encased; rounded shapes at the top of the egg cases indicate the stages |
| 0 | 1/2 Dose | 0 | 20 | 0 | Either in 8 or 16 cell stage. | ~ 1mm | Embryo is encased; rounded shapes at the top of the egg cases indicate the stages |
| 0 | Full Dose | 0 | 20 | 0 | Either in 8 or 16 cell stage. | ~ 1mm | Embryo is encased; rounded shapes at the top of the egg cases indicate the stages |
| 1 | Control | 0 | 20 | 0 | All in 72 hr stage. | ~ 2 mm | Eyes have behun to develop, retina pigmentation is relatively dark. |
| 1 | 1/2 Dose | 1 | 19 | 0 | All in 72 hr stage. | ~ 2 mm | Eyes have behun to develop, retina pigmentation is relatively dark. |
| 1 | Full Dose | 2 | 18 | 0 | All in 72 hr stage. | ~ 2 mm | Eyes have behun to develop, retina pigmentation is relatively dark. |
| 4 | Control | 5 | 1 | 14 | Most in early larvae stage. | ~ 4 mm | Longer tails, mobile, mouths are protruding/more noticeable, pectoral fins are developing. |
| 4 | 1/2 Dose | 0 | 1 | 18 | Early larvae stage. | ~ 3 mm | Longer tails, fin developing, mouth more noticeable, some are deformed with more curved shape |
| 4 | Full Dose | 3 | 0 | 15 | Early larvae stage. | ~ 3 mm | Deformities are showing (curved body instead of straight), but otherwise similr to 1/2 dose |
| 7 | Control | 9 | 0 | 5 | 72 hour stage | ~1 mm | Long tail, dark eye pigmentation, fins have developed for the most part, eyes are moving, little fins are developing and moving near the fish's head, yolk absorbed, slightly protruding mouth, moving back and fourth (using fins to move), dark tail pigmentation, tail is thick |
| 7 | ½ Dose | 4 | 0 | 14 | 72 hour stage | ~1 mm | Skinnier tail than control; very dark eye pigmentation, fin has developed for the most part, slightly protruding jaw, more fidgety (keeps running into side of plate) swimming side to side using tail & fin, no yolk, no eye movement, slight tail pigmentation. |
| 7 | Full Dose | 8 | 0 | 7 | 72 hour stage | ~1 mm | Mobility is very spastic (using fins and tails to move side to side, but not moving very far); end of tail is propelling fish forward; limited mobility; light pigmentation of tail; no eye movement and eyes seem significantly smaller & eyes are completely black; no yolk and pectoral fins are really skinny; flat jaw (not protruding). |
| 11 | Control | 5 | 0 | 0 | > 72 hour stage | ~2 mm | All dead; string-like pieces floating around, mold at center of plate, particles floating around plate. |
| 11 | ½ Dose | 14 | 0 | 0 | > 72 hour stage | ~2 mm | None alive, there are string-like particles floating around the plate & molds growing. |
| 11 | Full Dose | 7 | 0 | 0 | > 72 hour stage. | ~2 mm | All dead; string-like pieces and moldy caracases/particles floating around the plate. |
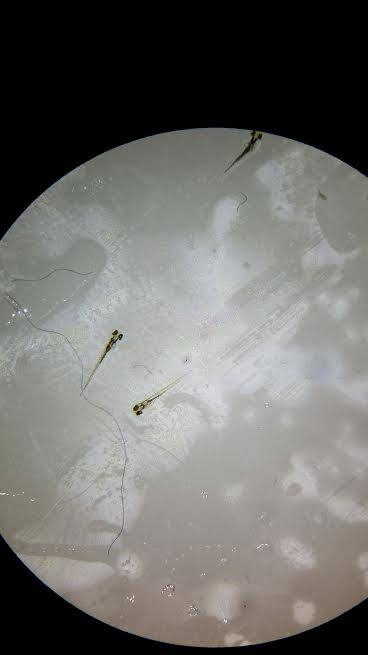
Above is an image of the Zebrafish on Day 1.
Above is an image of the Zebrafish on Day 4.
Above is an image of the control Zebrafish on Day 7.
Above is an image of the Zebrafish treated with the full dose on Day 7. Note the eye, body, and tail pigmentation.
Above is an image of the curved tail of the Zebrafish in the half dose on Day 7.
One of the mold-like substances found in stead of Zebrafish on Day 11 on the full dose plate.
One of the Zebrafish from Day 7 was preserved in formaldehyde. It appears as follows:
It is hypothesized that higher concentrations of fluoride will negatively effect the Zebrafish, as demonstrated by what was written in the paper. This hypothesis was found to be partially correct. While the presence of fluoride certainly negatively affected the Zebrafish in the half dose and full dose plates negatively, in the end they all died around the same day. This death came earlier than expected in all of the fish, and is perhaps due to the fluoride but could also simply have had to do with evaporation of water in all the plates or some sort of nutritional deficit in the fish. In general, though, some of the adverse effects described in the paper - a decrease in length and decreased mobility - were certainly true in those fish treated with the fluoride, and these defects were more present in the fish in the full dose solutions versus the half dose solutions.
2.20.15 Excellent lab book entry. Good description of invertebrate analysis and data. Good food web, a little small, hard to read. Well organized, I particularly like the contents section at the top of your notebook. SK
16S PCR Sequencing - February 26, 2015
The purpose of the PCR sequencing was to amplify the 16S rRNA gene found on bacteria. This sequence is unique to each separate bacteria, which makes it possible to distinguish between different organisms and identify those organisms in the transect.
Upon identifying the bacteria found in the Hay Infusion Culture via the agar plates, a polymerase chain reaction (PCR) was set up. One colony from each the tetracycline plate and plain agar plate was chosen for two separate PCR amplifications. Each colony was placed into 100 microliters of water in a sterile tube and was denatured for 10 minutes on a 100 degrees C heat block. The samples were centrifuged at 13,400 rotations per minute for 5 minutes. During this, 20 microliters of a primer/water mixture was added to a PCR tube and then mixed with a PCR bead. Five microliters of the supernatant from the centrifuged sample was transferred to the tube and then placed in a PCR machine. After one week, the PCR products were run through an agarose gel.
Because the PCR sequence this lab group turned out to have a purified product, a DNA sequence was found for each of the two bacteria, as follows:
MB47-For_16S_G06.ab1 NNNNNNNNNNNNNNNNNCTNNNCNTGCAGCCGAGCGGTAGAGATTCTTCGGAATCTTGAGAGCGGCGCACGGGTGCGGAA CACGTGTGCAACCTGCCTTTATCAGGGGAATAGCCTTTCGAAAGGAAGATTAATGCCCCATAATATATCATATGGCATCA TTTGATATTGAAAACTCCGGTGGATAAAGATGGGCACGCGCAGGATTAGATAGTTGGTAGGGTAACGGCCTACCAAGTCA GCGATCCTTAGGGGGCCTGAGAGGGTGATCCCCCACACTGGTACTGAGACACGGACCAGACTCCTACGGGAGGCAGCAGT GAGGAATATTGGACAATGGGTGAGAGCCTGATCCAGCCATCCCGCGTGAAGGACGACGGCCCTATGGGTTGTAAACTTCT TTTGTATAGGGATAAACCTACCCTCGTGAGGGTAGCTGAAGGTACTATACGAATAAGCACCGGCTAACTCCGTGCCAGCA GCCGCGGTAATACGGAGGGTGCAAGCGTTATCCGGATTTATTGGGTTTAAAGGGTCCGTANGCTGATGTGTAANTCANTG GTGAAATCTCACANCTTANCTGTGAAACTGCCNTTGATACTGCATGTCTTGAGTGTTGTTGAANTANCTGGAATAANTNN GTANCAGTGAAATGCCTANATATTACTTNNANCACNANGTGCTAANGCANGTTGGTANNCCNCNACTGACNCTGATNGAG NAAANCNTGGGNNAGCGAACANAANTNNATACCCTGGGGNNGTNNNCNNNAANNAANCTNANTCNNTTTTTNTCTTTCTC TTNCNNATACANNNNNANCCGANAAGNTNGCCNNCTNCCGGGTGGTGTTCTCCNTNNTNNNGATGNNNTCNNCTNNNNNN NNNNNCNGCCCCCCCNCAANNATTTNTANANNNNTATANNNTNNNANANCNNGCGGCCCCCTNTNTAANNGGNNNNGGGG GAGNNNNNNGNNNNNGTTTTCTATTATATNTNNNNCTNTNNNCCNCNNGNNCNGGGGGGGTTGTNTCTCCCNNCCAGAAC NNAANGANANTNTNCNNCANCAGCCNNNN
MB48-For_16S_H06.ab1 NNNNNNNNNNNNNNGCNNANNNTGNNANNNNNGCGGTANGANGGGANGCTTGCTCTNNGATTCAGCGGCGGACGGGTGAG TAATGCCTAGGAATCTGCCTGGTAGTGGGGGACAACGTTTCGAAAGGAACGCTAATACCGCATACGTCCTACGGGAGAAA GCAGGGGACCTTCGGGCCTTGCGCTATCAGATGAGCCTAGGTCGGATTAGCTAGTTGGTGAGGTAATGGCTCACCAAGGC GACGATCCGTAACTGGTCTGAGAGGATGATCAGTCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAG TGGGGAATATTGGACAATGGGCGAAAGCCTGATCCAGCCATGCCGCGTGTGTGAAGAAGGTCTTCGGATTGTAAAGCACT TTAAGTTGGGAGGAAGGGCATTAACCTAATACCTTGGTGTTTTGACGTTACCGACAGAATAAGCACCGGCTAACTCTGTG CCAGCAGCCGCGGTAATACAGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCGCGTANGTGGTTTGTTAAG TTGGATGTGAAAGCCCCGGGCTCAACCTGGGAACTGCNTTCNAAACTGNCNAGCTAGAGTATGGTANAGGGTGGTGGAAT TTCCTGTGTAGCNNTGAAATGCGTAGATATANGAANGAACACNNNGTGGCNAAGCGGACCACCTGNACTGATACTNACAC TGANNTGCGAAANNNTGTGGANCAAACANNATTANGATNNCCTNNAGTCCACNGCCNGTANACNNNNTCAACTANCNNNN NNAGCNCTTNANNTGTTANTGNCGCNNCTAACNCATTAANTNNCNNCGCTGGNTNNGTAGNAGNCCNCGNCCGTTAGNNC TNNNNNNGGAGTTNANNGGNGCCNNGCACAAGCNACTGNAGCAGGGNGGGNTGTAGTTCCNAANNNNNNACNAAAAANNN NNACCCNGNCCCTNGGNATNNAANNNAGNNNNNGAGGNNNNNNAANNNGNGNNNNGGNTGNNNNCNNNGGAAANNNNACC ANNNGNNNATGGTNGGNNNNNNNCNNCANNNNNNCNANCCCNNNNNNN
Conclusion: Using an online website, the lab group was able to determine the species of the two bacteria. The first sequence pointed to the bacteria being a Chryseobacterium, and this is consistent with the observations made in the lab; the chryseobacteria was gram negative, circular, and moves in a gliding motion. These bacteria can be found in many different organisms and have many varieties, including raw milk. The bacteria of the second sequence matched the Pseudomonas aeruginosa. This also matches the observations made; the bacteria is gram negative, coccus or bacillus in shape, and has some motility.
Invertebrates - February 12, 2015
The purpose of this lab is to understand the importance of invertebrates and to understand the evolution of simple systems into more complex ones, which is how the vertebrate organ system developed from these simple organisms.
Procedure 1: Each lab group began by observing three different types of worms - the acoelomate Planaria (a flatworm), a nematode (a roundworm), and the coelomate Annelida (an earthworm) - under microscopes. Observing these three invertebrates was meant to demonstrate how the worms' different forms of movement related to their body structure.
Procedure 2: In this portion of the lab, students were to observe example invertebrates from the five major classes: arachnida, diplopoda, chilopoda, insect, and crustacea. Students were meant to differentiate between the organisms based on their body parts and the number of appendages they each had.
Procedure 3: In this portion of the lab, students were sent to observe the invertebrates found in the Berlese Funnel. After breaking down the funnel, each lab group poured the top 10-15 mL of liquid and organisms into a petri dish, and the remaining liquid into another. Then, using a dissecting microscope, each lab group attempted to identify the class of Arthropoda invertebrates using a key. Observations were recorded in a table.
Procedure 4: This final part of the lab - and a culmination of the study - had students consider the vertebrates that pass through the transect, whether those vertebrates be a niche for the organisms or simply part of the niche. Students identified five of these organisms, determined their classification, and considered the biotic and abiotic characteristics of their particular transects that would benefit each species. Then, a food web was created based on every organism observed in the transect.
All three of the worms moved by contracting and releasing their muscles. Though evolutionarily they might appear to be different, their movement in this regard seems to be similar.
The table below provides a detailed description of the five invertebrates found in the Berlese Funnel:
| Organism | Length (mm) | # in Sample | Description of Organism |
| Springtail (Phylum: Athropoda; Class: Hexopoda) | Appx. 10 mm | 2 | Antannea preseent; hairlike features; 3-6 legs; segmented body |
| Soil Mite (Phylum: Arthropoda; Class: Arachnida) | Appx. 1 mm | 20 | Very small, circular, non-segmented body, many present |
| Pseudoscorpions (Phylum: Athropoda; Class: Arachnida) | Appx. 8 mm | 4 | Very small, segmented body, two piercers preesent |
| Beetle Larvae (Phylum: Arthropod; Class: Insecta) | Appx. 5 mm | 1 | Small, circular in shape, few legs/hairlike features |
| Scale or Aphid (Phylum: Arthropoda; Class: Insecta) | Appx. 20 mm | 1 | Large in size, striped, harilike features, 6 legs, long antaneea |
The following are photos of the organisms found in the transect:
In the image above, the large organism is the Springtail. Above it is a Soil Mite.
In the image above, a Pseudoscorpion is shown to the left, and on the right is a second Soil Mite.
Pictured above are two beetle larvae.
Pictured above is a scale or aphid.
Below is a chart that classifies and describes the various vertebrates that are predicted to live in or pass through the transect. Abiotic and biotic factors of the transect that the organisms could find beneficial are listed below.
| Vertebrate | Classification (Phylum/Genus/Species) | Beneficial Biotic | Beneficial Abiotic |
| Chipmunk | Chordate/Tamias/T. alpinus | Lots of seeds of plants in the planters to be eaten | Chipmunks can burrow into the soil |
| Deer | Chordata/Odocoileus/O. virginianus | Lots of vegetation to consume | Low fences/planters are easy to climb over, mulch is not difficult to tread on |
| Hawk | Chordata/Buteo/B. jamaicicenis | Can eat organisms such as rats that frequent the transect | Dead weeds at the top of transect can be used to build nest, as can branches/branch-like pieces of mulch |
| Rat | Chordata/Rattus/R. norvegicus | Can burrow into the plants for shelter & consume them if hungry | Can burrow into the soil/under the planters for safety |
| Robin | Chordata/Turdus/T. migratorius | Lots of seeds of plants in the planters to be eaten | Dead weeds at the top of transect can be used to build nest, as can branches/branch-like pieces of mulch |
Below is a food web of all the organisms - vertebrates, invertebrates, and plants - found in the transect. The food web describes the passage of energy from each level of organism to the next.
Results: The observing of and classification of invertebrates found in the Berlese funnel through this experiment led to a greater understanding of the biodiversity found in such a small area of land. The analysis of the vertebrates provided this same understanding. Creation of the food web allowed a look at this particular community, or all the intersections of different species in this one particular geographical area. The leveling of the food chain allowed for the separation of organisms into their separate trophic levels, or the position an organism occupies in a good chain. The producers - organisms that make their own food - were found at the bottom, and following them were primary, secondary, and tertiary consumers. The energy being transferred from each trophic level decreases as the levels increase, which could account for why most food webs appear to have more producers than tertiary consumers. It is unknown whether or not this particular transect has reached its carrying capacity, or the maximum number of organisms that can live in the area.
- Esha B. Dholia 02:19, 18 February 2015 (EST):
2.20.15 Excellent notebook entry. SK
Plantae & Fungi - February 5, 2015
Purpose: The objectives of this lab were to understand the various characteristics and diversity of plants, and to appreciate the function and role fungi play in everyday life.
Procedure 1: Each lab group obtained two to three Ziploc bags and proceeded to their transects. In the first bag, each group was required to collect a leaf litter sample, which would be composed of about 500g of dead leaves and plant matter. The bag should have contained about 80% leaves and only 20% of the crumbly soil. The second bag should have contianed a representative sample from five different plants in the transect in a way that would be minimally damaging. This included collecting any seeds, pine cones, and flowers in the transect, and would be used in the lab to identify the major group and genus of each of the five samples.
Procedure 2: In order to understand what genus the plant samples are from, each lab group observed different characteristics of the plant, including plant vascularization. Each group first observed a moss and a lily plant stem. They then observed a cross section of the lily stem, looking specifically for xylem and phloem layers. Based on these observations, each lab group then was to characterize the vascularization in each of the plants from their transects.
Procedure 3: Another characteristic the lab groups observed was the presence of specialized structures in the plants. The lab groups were to describe the shape, size, and cluster arrangement of the leave from their transect plants, as well as the attachment sites of leaves on these plants and evidence of leaves in the area via leaf litter.
Procedure 4: A third characteristic the lab groups observed was the mechanism of plant reproduction. The goal of this portion of the lab was to determine the way in which the plants in the transect reproduce. After observing moss, a lily flower, and seeds, each lab group was to observe seeds collected from their transects to identify if they were monocot or dicot, and whether or not there was evidence of flowering or spores. In this particular lab group, which was responsible for transect 4, no seeds were found for fear of disrupting the environment.
Procedure 5: This portion of the lab called for observing fungi and identifying the different structures and characteristics that make fungi important. Each lab group was responsible for looking at some of the samples and deciding whether or not the organisms were fungi, and which of the three groups they belonged to.
Procedure 6: The final portion of this lab was setting up a Berlese Funnel to collect invertebrates from the transect. The leaf litter Ziploc bag was to be used in this portion of the lab. Each lab group poured 25 mL of a 50/50 ethanol-water solution into a 50 mL conical tube. After fitting a piece of screening material into the bottom of the funnel, the leaf littler sample was carefully put in the top of the funnel. The funnel was then set up on a ring stand so it would be held in the tube with the ethanol, and then the base of the funnel was parafilmed so the ethanol would not evaporate. A lighted watt lamp was placed above the funnel with an incandescent lightbulb about 1-2 inches from the top of leaf litter, and everything was covered with foil. The Berlese funnel was left on the lab bench for one week for the invertebrates to grow.
The following table contains information about the five plants collected from Transect 4, including each plant's method of vascularization, reproduction, specialized structure, shape, and size.
| Transect Sample Plants | Location & # in Transect | Description (Size & Shape) | Vascularization | Specialized Structures | Mechanisms of Reproduction |
| 1 | Third planter box, brussel sprouts | Brussels sprout leaf, very large (19 cm from stem to top, veins are crisscrossed, thick stem, leaf has waxy, wavy, hard texture) | Xylem & phloem | Chlorophyll, ovary, & ovules | Alternation of generations |
| 2 | First planter box, kale | Thick stem, crisscrossed veins, curly/wavy leaves, hard in texture, large sized leaves, 11 cm from root to tip | Xylem & phloem | Guard cells, stomata, chlorophyll | Alternation of generations |
| 3 | Fourth planter box, lettuce | Small stem, flat veins are parallel to one another, soft texture, smaller leaves, 9.5 cm from top of leaf to bottom | Xylem & phloem | Guard cells, stomata, chlorophyll | Alternation of generations |
| 4 | Second planter box, grass | Short, similar-sized roots, one part red & other part green, no visible veins | Xylem & phloem | Chlorophyll, stomata, guard cells | Alternation of generations |
| 5 | Fourth planter box, weed | Weed, many leaves, crisscrossed veins, small in size (about 2.4 cm sized leaves), small ridges and rounded leaves | Xylem & phloem | Chlorophyll, stomata, guard cells | Alternation of generations |
The following are photos of the five different samples collected from Transect 4.
Sample one is the brussels sprout leaf collected from one of the planters.
Sample two is one of the kale leaves collected from one of the planters.
Sample three is a lettuce leaf collected from one of the planters.
Sample five is a weed collected from one of the planters.
The specimen observed in Procedure 5 are classified in the table below:
| ' | Bread Mold | Rhizopus Nigricans | Mushroom |
| Fungi? | Yes | Yes | Yes |
| Classification | Ascomycata | Zygomycata | Basidiomycota |
The following is an image of Rhizopus stolonifer, which was found to be a zygomycata. It can be classified as a fungi because it has many sporangia, which in fungi are the site of meiosis and asexual reproduction (http://www.britannica.com/EBchecked/topic/222357/fungus/57965/Sporophores-and-spores#ref519195).
Observing the various plants in Transect 4 allowed for a greater understanding of the immense diversity of plants. Most of the plants in Transect 4 appeared to have similar methods of reproduction, specialized structures, and methods of vascularization, which makes sense seeing as they are all garden plants and being grown in similar environments, which accounts for their many similarities. Future observation of the soil and leaf litter left for development in the Burlese funnel will be interesting and assist in making greater sense of the transect and its organisms.
--Esha B. Dholia 21:20, 11 February 2015 (EST)
2.10.15 Excellent notebook entry. Plenty of detail in methods and observations. Nice images and good formatting. SK
Observing Antibiotic Resistance & Identifying Bacteria with DNA Sequences - January 29, 2015
The purpose of this lab was to understand the different characteristics of bacteria, observe and understand antibiotic resistance in these bacteria, and understand how DNA sequences can be used to identify different species. In the previous lab, eight agar plates, four with the antibiotic tetracycline, were plated with various dilutions of the bacteria cultures for each group's transect. It is hypothesized bacteria will have grown on the plates, but not archaea, because archaea only tend to grown in extreme environments and these plates were simply left at room temperature for a week. It is also hypothesized that the hay culture will have changed in appearance sine last week. This change in appearance can be attributed to the change in time; as the weeks have progressed, bacteria and other living organisms have had time to grow and change in appearance.
Procedure 1: Each lab group retrieved their eight agar plates inoculated from the Hay Infusion Culture to count the total number of colonies per plate. Depending on the plate and whether or not tetracycline was present or not, each lab group could expect to see a variety of different numbers of cultures on their plates.
Procedure 2: Each lab group then considered the differences in the number of colonies on plates with and without the antibiotic, and attempted to determine the mechanisms which could have caused resistance in the bacteria to the tetracycline.
Procedure 3: Each lab group was to observe four different samples of microorganisms, two from the nutrient agar plates and two from the nutrient agar plus tetracycline plates. For each sample, a wet mount and a gram stain was to be prepared; then, the samples were to be observed using a high-power lens or the 100x oil immersion technique. To prepare the wet mount, a loop was sterilized over a flame with alcohol and used to scrape up one of the growths off the surface of one of the agar plates. This small colony was then mixed with a drop of water on a slide, covered with a cover slip, and observed under the microscope. Because it was difficult to see the organisms because they were not stained, the oil immersion at 100x was an option to see the bacteria better. The cell shapes and motility of the organisms were determined via the wet mount. To prepare a gram stain, the loop was sterilized over a flame and used to scrape up a small amount of the growth from the agar. It was mixed with a drop of water on a slide. The area beneath the sample was circled with a red wax pencil, and the slide was labeled with the name of the agar plate. Then, the slide and its contents were heat fixed by passing the slide through a flame three times with the bacterial smear side up. In a staining tray, the bacterial smear was covered with crystal violet for one minute, and then rinsed off using a wash bottle filled with water. The smear was then covered with Gram's iodine mordant for one minute, and again washed with water. To decolorize the bacterial smear, a rinse with 95% alcohol was applied to the slide for 10-20 seconds. The smear was then covered with a safranin stain for 20-30 seconds and rinsed with water. The excess water was blotted away with a kimwipe and the slide was allowed to air dry. Then, the gram stained sample was observed under the microscope. The gram stain procedure and the wet mount procedures were used for all four samples.
Procedure 4: Each group characterized four bacteria from the Hay Infusion Culture, two from each of the two types of plate. One bacteria from each of the two plates was selected for PCR analysis to amplify the 16S rRNA gene, a sequence that is specific to each species, which will occur in the next lab. The colonies were each transferred to 100 microliters of water in two different sterile tubes. They were incubated at 100 degrees Celsius for 10 minutes in a heat block, and then centrifuged for 5 minutes at 13400 rpm. During the centrifugation, 20 microliters of primer were added to a labeled PCR tube, and mixed to dissolve the PCR bead. Finally, 5 microliters of the supernatant from the centrifuged sample were transferred to the 16S PCR reaction. The tubes were then placed in the PCR machine.
Data & Observations: Before carrying out Procedure 1, it was noted that the Hay Infusion Culture did not have any scent, but was very brown and the film that had persisted on the sides of the culture's container was present and perhaps thicker than it had previously been. The following table contain the results of the 100-fold serial dilutions results:
| Dilution | Agar Type | Colonies Counted | Conversion Factor | Colonies/mL |
| 10^-3 | Nutrient | 37 | x 10^3 | 37000 |
| 10^-5 | Nutrient | 17 | x 10^5 | 1700000 |
| 10^-7 | Nutrient | 1 | x 10^7 | 10000000 |
| 10^-9 | Nutrient | 0 | x 10^9 | 0 |
| 10^-3 | Nutrient + tet | 14 | x 10^3 | 14000 |
| 10^-5 | Nutrient + tet | 0 | x 10^5 | 0 |
| 10^-7 | Nutrient + tet | 0 | x 10^7 | 0 |
| 10^-9 | Nutrient + tet | 0 | x 10^9 | 0 |
For the morphology observations, the 10^-3 plates with and without tetracycline were chosen. A yellow culture and a white culture were chosen from each of these plates to represent bacterial diversity. The following are photographs of the bacteria without the gram-staining:
The 10^-3 culture, before any tests were ran.
The 10^-3 culture plus tetracycline, before any tests were ran.
A white colony from the 10^-3 plate was sampled and observed under a microscope without any staining at 40x. They are very small and difficult to see.
A white colony from the 10^-3 plate with tetracycline was sampled and observed under a microscope without any staining. The string-like arrangement of the cells is apparent here.
A yellow colony from the 10^-3 plate was sampled and observed under a microscope without any staining. The cells appear raised and in string-like arrangements, and have rod shapes.
The following are photographs of the bacteria after the gram-staining:
The white 10^-3 colony after staining. The violet color indicates peptidoglycan is present in the cell walls.
The white 10^-3 colony with tetracycline after staining. Again, the violet color indicates peptidoglycan is present in the cell walls.
The yellow 10^-3 colony after staining. Because the bacteria appear pink, it can be inferred that they are gram-negative and do not have peptidoglycan.
The yellow 10^-3 colony after staining. There are not many bacteria present in this view; however, because they are violet, it is known that peptidoglycan is present in their cell walls.
The following table synthesizes all the information about the morphologies of the bacterial colonies and cells observed, both before and after staining.
| Colony Label | Plate Type | Colony Description | Cell Description | Gram +/- |
| White | 10^-3 | Approximately. .5 cm across, small, white. Entire & undulated edge. Flat elevation. Glistening, smooth surface. Circular. | Cells are vibrating, circular, and very close together. About 5 micrometers at 40x. | + |
| Yellow | 10^-3 | Colony is circular, smooth and shiny. Irregular shape, filamentous edges, and wrinkled surface. Flat elevation, approximately. .5 cm across. | Cells are rod-shaped and arranged in long strings. They appear to look like raised palisades. Cells are not motile, though this could have been due to lack of water ion the slide. About 1 micrometer at 100x. | + |
| White | T10^-3 | Circular colony shape. Edges are entire, surface is smooth and glistening, elevation is convex. Small, approximately .2 cm in diameter. | Cells are arranged in string-like patterns and are mainly circular, though some are ovular. They bear resemblance to streptobacilli. About 5 micrometers at 40x. | - |
| Yellow | T10^-3 | Circular colony shape. Edges are entire, surface is smooth and glistening, elevation is convex. Approximately .5 cm in diameter. | Coccus in shape, circular, individually dispersed through culture and not in groups, not motile. There are not many cells present on the slide. About 4 micrometers at 40x. | + |
Conclusions & Future Directions:
After examining the colony types between the plates with and without the antibiotic, it was found that the plates with just the broth had more colonies than the plates with the broth and the tetracycline. This is because of bacterial resistance and natural selection. Only some of the bacteria on the tet plates were resistant to tetracycline; those that were not did not survive, but those that were survived to reproduce and form the colonies seen on the plates. There was one mold growing on the 10^-3 plate with tetracycline, but all the others were bacterial cultures. Bacteria that are resistant to tetracycline employ one of three mechanisms: tetracycline efflux, ribosome protection, and tetracycline modification (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC358256/). Each of these mechanisms has to do with the genotype of a particular bacteria, and is passed down from generation to generation genetically, allowing for natural selection and consequently evolution to do their work. Overall, tetracycline decreases the number of bacteria by weeding out the ones that are not resistant to the antibiotic. *ED:
2.4.15 Excellent notebook entry. Included lots of detailed description of Hay Infusion and location of protists observed. Good description of protists and identification. SK
Hay Infusion Culture Observations - January 22, 2015
Purpose: The purpose of this lab was to practice using a Dichotomous key to identify unknown organisms, and then to use this knowledge as well as an understanding of the characteristics of algae and protists to identify and examine the algae and protists from each group's own transect.
Procedure I - Using the Dichotomous Key: To become comfortable with the Dichotomous lab group was to make a wet mount of known organisms and observe them under the microscope at 40x objective. Then, a single organism had to be focused on and described by its size, shape, and various features. A Dichotomous key was then obtained and used to confirm the identity of the known organism by following the diagrams.
Procedure II - Hay Infusion Observations: Each group brought the culture back to their work areas to note any scents and describe the cultures' appearances. Then, each group took two samples from different niches of the culture, making sure to include some plant matter, and prepared two different wet mounts. Then, observing the slides under the microscopes, each group used the Dichotomous key to determine which algae and protists were present. The group drew pictures of the organisms observed and recorded their size. Each group was responsible for finding at least 3 different organisms from each of the two niches.
Procedure III - Preparing & Plating Serial Dilutions: Upon completing the Hay Infusion, a serial dilution was to be plated, which would allow prokaryotic organisms and possibly fungi from the culture to be observed. To do this, 4 tubes of 10 mL sterile broth were labeled with 10^-2, 10^-4, 10^-6, and 10^-8. Four nutrient agar plates and four nutrient agar plus tetracycline plates were then obtained. One plate from each of the groups was labeled with 10^-3, 10^-5, 10^-7, and 10^-9, with the tetracycline plates labels prefaced with "tet." Then, using a micropipettor, 100 microliters of the culture was added to the broth in the 10^-2 tube. The tube was then mixed thoroughly. One hundred microliters of broth from the 10^-2 tube was added to the 10^-4 tube, and swirled well. This was repeated to make the 10^-6 and 10^-8 dilutions. To plate these dilutions on the agar plates, 100 microliters from the 10^-2 tube was pipetted onto the plate labeled 10^-3. The sample was carefully spread on the plate, and the procedure was repeated on the 10^-3 plate containing tetracycline. This process was repeated to add the 10^-4 dilution to the 10^-5 plate, the 10^-6 dilution to the 10^-7 plate, and the 10^-8 dilution to the 10^-9 plate. The plates were placed on a rack to incubate at room temperature for one week. The process is depicted below.
Data & Observations: Upon bringing the culture back to the table, it was noted that the culture had no scent and had taken on a brownish color. There was dirt floating in the culture at the bottom, and a thin brown film coated the top of the culture as well as the sides of the jar it was in, but there were no apparent signs of life. The first sample was taken from the top of the culture, where a cloudy film of dirt was noted. Three different organisms - chilomonas, colpidium, and paramecium aurelia - were observed. Each of the organisms appeared to be moving quickly and had some sort of mechanism to propel it along. The chilomonas and paramecium both had cilia all over their body, while the colpidium had two flagella. The three organisms were protozoa, because they did not have any green coloring and therefore could not have been photosynthetic organisms. The colpidium was oval-shaped and 51 micrometers, the chilomonas was 22 um, and the parameciumm was 156 um. The following is a picture of the site where the paramecium was seen:
The second sample taken came from the soil at the bottom of the culture. Here, a peranema, arcella, and paramecium bursaria were identified. The peranema and paramecium were both more motile than the arcella, which was creeping slowly around the sample. While the peranema and arcella were colorless and therefore could be presumed to be protists, the paramecium itself was green in color, meaning it is photosynthetic. This makes sense, as it was found close to the clump of dirt in which plants may have been growing. The arcella was 63 um, the paramecium was 156 um, and the paranema was 45 um. Pictured below is the sample of soil where the peranema was seen:
Conclusions: This lab demonstrated the diversity of organisms in niche, even though the niche was so small. Organisms differ where they are, if they're closet to plant matter or not. If organisms are closer to plant matter, it's likely they themselves are photosynthetic algae and produce their own food. As with the organisms in this niche, because much plant matter was not present, the observed organisms were all protists. The arcella, like all the organisms, satisfies all the needs of life: it has to acquire energy to survive and reproduce, which it does through consumption of nutrients externally. It's a unicellular organism and has its own genetic information, which allow it to stay alive. The arcella reproduces asexually by replicating its genetic information and then passing down identical versions of it to offspring, and overtime the arcella likely evolves, based on mutations in its genes and how adapted the protist is to its external environment. If the culture were to grow for another two months, it's possible plants would actually grown, seeing as the soil sample was taken from a garden, but it's also possible the soil would continue to sit and disintegrate at the bottom of the culture. Those communities within the culture that are reliant on the large chunks of soil at the bottom would be most affected by this and would no longer have the soil as a mechanism to cope. Selection would weed out those organisms that have low fitnesses and are not well-adapted to the environment.
- Esha B. Dholia:
1.27.15 Good first entry. Missing some information eg. the Hay infusion set up. The transect was 20Ft by 20Ft. We will work on uploading pictures this week. SK
Initial Transect Observations & Notes - January 15, 2015
Purpose: This lab is an ongoing experiment, in which a particular transect of land and its ecological diversity on the American University will be observed by a group of students for several weeks. The purpose of this lab is to take note of the features of that particular transect in order to begin conceptualizing the immense diversity present within a single ecosystem.
Materials & Methods: The group was assigned transect 4, one of the five 20x20 ft parcels of land students in the class will be responsible for observing over a few weeks. In terms of what could be variable in later observations of this transect, the weather should certainly be taken into consideration; because data is being gathered in the winter, the organisms observed on the transect and their number could vary from what's seen in this same transect of land during different times of the year. The group was responsible for noting all aspects of this transect of land, including its situation, biotic and abiotic factors, and other characteristics. Upon completing the observations, the group collected a 50 mL sample of soil and ground vegetation in a sterile conical tube - meant to represent the transect - to make a Hay Infusion culture.
Data & Observations: This lab group was assigned transect four, which is located within the American University community garden next to the tennis courts, towards the back of the campus. Transect 4 is located in a relatively relatively isolated area, though it is certainly well-kept and tended to often. The transect is located on a flat piece of land, and is fenced off. Pictured below is an aerial-view diagram of the transect.
The four beds appeared to be well-watered, though they did seem to be exhibiting any signs of exceptional plant growth. Certain vegetables had better growth than others.
The biotic, or living, factors found in this transect include the vegetables being grown, birds, weeds, earthworms, and ants. The vegetables were found growing in the four vegetable beds, and the weeds were found in these beds as well as in the surrounding soil and mulch. Birds were observed flying above the transect and it is presumed they consume the seeds and leaves of the vegetation in the garden. Ants and earthworms were found in the bed of the planters, and ants could likely also have been found in the mulch.
The abiotic, or nonliving, factors observed include the mulch, the planters, the irrigation system, the soil, and the water. Mulch, made of a mix of tan bark and other dead organic matter such as dead leaves and plants, is present throughout this transect, and is on the ground surrounding all the planters. The planters themselves are made of wood, and house all of the vegetation purposefully grown on this transect. The irrigation system is a series of pipes that runs throughout this transect and is responsible for bringing water to the planters. Soil is found in the beds in which the plants are meant to grow, and water is nearly everywhere, including in the soil and in the mulch. The sample collected for the Hay Infusion included soil from each of the four beds in the transect as well as mulch from both around the planters as well near the dead plants towards the back.
Conclusions & Future Directions: Upon closer examination of transect 4 - which had initially appeared to simply be a community garden - it's quite clear that there is an immense amount of life and diversity living and thriving in this environment. The Hay Infusion will further reveal what sort of protists and bacteria have a home in this transect, as well. In order to further understand the diversity of life on campus, it would be interesting to compare this transect of land with the four others, and attempt to comprehend the variance in life present on the American University campus.