User:Floriane Briere/Notebook/CHEM-496/2012/04/04: Difference between revisions
(→Data) |
mNo edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 11: | Line 11: | ||
==Objective== | ==Objective== | ||
Today's objective is to synthesize Gold NPs again, using different stock solutions. These solutions we'll be used as control for our further analyses. Moreover, we're also going to determine the dye/BSA ratio; to do so we'll use Beer's law and our UV spectrum. | |||
== | ==Protocol== | ||
* 166 ratio solution: | |||
# 8ml of water | |||
# 1ml of BSA (17.7µM) | |||
# 1ml of Gold (2.9mM; prepared on the 3/21) | |||
* 70 ratio solution: | |||
# 8.5ml of water | |||
# 1ml of BSA (17.7µM) | |||
# 0.42ml of Gold (2.9mM; prepared on the 3/21) | |||
==Data== | ==Data== | ||
One of our objective is to determine the number of Lysine (on the BSA molecule) which are bound to a dye molecule. To do so, we need to evaluate the concentration of dye in our solution and the amount of BSA protein. | |||
The amount of dye in each solution can be determined using UV spectrum; the dye in solution has a known-concentration which will allow us to find the molar absorptivity of the dye (at 602nm and 625nm; Gold particles also absorb around 550nm and we want to lower this effect as much as possible). | |||
* Determination of the molar absorptivity of the dye (at 602nm) using a known-concentration solution. | |||
** Determination of the concentration of the dye in solution (C). | |||
The dye in solution has been prepared on the 21/2 using: | The dye in solution has been prepared on the 21/2 using: | ||
m = 1mg of dye | m = 1mg of dye | ||
5ml of water + 80µL of DMSO => Total volume = V = 0.0058L | 5ml of water + 80µL of DMSO => Total volume = V = 0.0058L | ||
MW of the dye = 573.51 g/mol | MW of the dye = 573.51 g/mol | ||
So, n = 10^-3/573.51 = 1.74* 10^-6 moles | So, n = 10^-3/573.51 = 1.74* 10^-6 moles | ||
And, C = (1.74* 10^-6)/0.0058 = 3*10^-4 M | And, C = (1.74* 10^-6)/0.0058 = 3*10^-4 M | ||
** Determination of the molar absorptivity of the dye using Beer's law. | |||
We are going to use the Absorbance at 602nm (which is the absorption value given by the dye manufacturer); at this wavelength, fluctuation should be the lowest. | We are going to use the Absorbance at 602nm (which is the absorption value given by the dye manufacturer); at this wavelength, fluctuation should be the lowest. | ||
Molar absorbtivities (ε, L/mol/cm) were determined using Beer's law: A = ε*l*C | Molar absorbtivities (ε, L/mol/cm) were determined using Beer's law: A = ε*l*C | ||
With A = absorbance at 602nm | With A = absorbance at 602nm | ||
l = width of the cuvette (1cm) | l = width of the cuvette (1cm) | ||
| Line 37: | Line 55: | ||
[[Image:4april-Absorbance table.png]] | [[Image:4april-Absorbance table.png]] | ||
The molar absorptivity of our dye is equal to = 3419 L.mol^-1.cm^-1 | The molar absorptivity of our dye at 602nm is equal to = 3419 L.mol^-1.cm^-1 | ||
(It's determined by doing the average of the three different ε values) | (It's determined by doing the average of the three different ε values) | ||
== | * Determination of the molar absorptivity of the dye (at 625nm) using a known-concentration solution. | ||
Molar absorbtivities (ε, L/mol/cm) were determined using Beer's law: A = ε*l*C | |||
With A = absorbance at 625nm | |||
l = width of the cuvette (1cm) | |||
C = 3*10^-4 M | |||
[[Image:4april-Absorbance table 625.png]] | |||
The molar absorptivity of our dye at 625nm is equal to = 2204 L.mol^-1.cm^-1 | |||
(It's determined by doing the average of the three different ε values) | |||
CONCLUSION: we determined a very low molar absorptivity; we found in the literature a more logical value; we'll use the literature value (42000 IU) for our further calculations. | |||
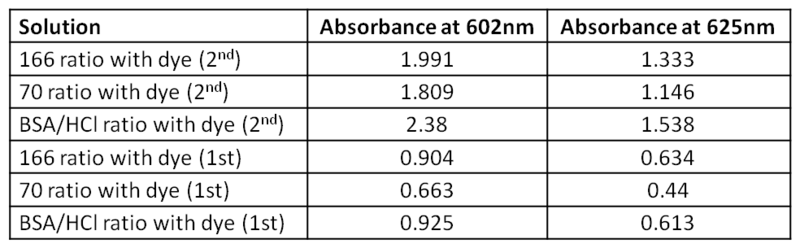
* Determination of the dye concentration in 166, 70 and BSA/HCl solutions (using 3/27 UV spectrum). | |||
[[Image:4-4-2012-3.jpg]] | |||
In this table are corrected value for Absorbance (Abs with dye - Abs without dye) | |||
According to these data, we can calculate the dye concentration using Beer's law. | |||
[[ | [[Image:4-4-2012-4.jpg]] | ||
SUMMARY: Determining Dye Concentration: | |||
# Using the UV-VIS of a known dye standard, the molar absorptivity of the dye was determined using Beers Law. The molar absorptivity value calculated from the UV spectra of the dye was too low, so used the value obtained in literature | |||
# Beer’s Law and the calculated molar absorptivity were used to determine the concentration of dye in all of the dye reactions. | |||
To account for the presence of gold nanoparticles and BSA solutions, corrected values for absorbance were used. The corrected values were acquired by subtracting the absorbance of the control solutions (166, 70, and BSA/HCl without dye) from the absorbance values obtained from the spectra for each trial. | |||
# We used the initial concentrations of BSA and assumed that no BSA had been lost during the experiment | |||
[[Category:Course]] | [[Category:Course]] | ||
Revision as of 12:56, 30 April 2012
 Chem-496 Chem-496
|
<html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> |
|
ObjectiveToday's objective is to synthesize Gold NPs again, using different stock solutions. These solutions we'll be used as control for our further analyses. Moreover, we're also going to determine the dye/BSA ratio; to do so we'll use Beer's law and our UV spectrum. Protocol
DataOne of our objective is to determine the number of Lysine (on the BSA molecule) which are bound to a dye molecule. To do so, we need to evaluate the concentration of dye in our solution and the amount of BSA protein. The amount of dye in each solution can be determined using UV spectrum; the dye in solution has a known-concentration which will allow us to find the molar absorptivity of the dye (at 602nm and 625nm; Gold particles also absorb around 550nm and we want to lower this effect as much as possible).
The dye in solution has been prepared on the 21/2 using: m = 1mg of dye 5ml of water + 80µL of DMSO => Total volume = V = 0.0058L MW of the dye = 573.51 g/mol So, n = 10^-3/573.51 = 1.74* 10^-6 moles And, C = (1.74* 10^-6)/0.0058 = 3*10^-4 M
We are going to use the Absorbance at 602nm (which is the absorption value given by the dye manufacturer); at this wavelength, fluctuation should be the lowest. Molar absorbtivities (ε, L/mol/cm) were determined using Beer's law: A = ε*l*C With A = absorbance at 602nm l = width of the cuvette (1cm) C = dye concentration (3*10^-4 M) The molar absorptivity of our dye at 602nm is equal to = 3419 L.mol^-1.cm^-1 (It's determined by doing the average of the three different ε values)
Molar absorbtivities (ε, L/mol/cm) were determined using Beer's law: A = ε*l*C With A = absorbance at 625nm l = width of the cuvette (1cm) C = 3*10^-4 M The molar absorptivity of our dye at 625nm is equal to = 2204 L.mol^-1.cm^-1 (It's determined by doing the average of the three different ε values) CONCLUSION: we determined a very low molar absorptivity; we found in the literature a more logical value; we'll use the literature value (42000 IU) for our further calculations.
In this table are corrected value for Absorbance (Abs with dye - Abs without dye) According to these data, we can calculate the dye concentration using Beer's law. SUMMARY: Determining Dye Concentration:
To account for the presence of gold nanoparticles and BSA solutions, corrected values for absorbance were used. The corrected values were acquired by subtracting the absorbance of the control solutions (166, 70, and BSA/HCl without dye) from the absorbance values obtained from the spectra for each trial.
| |