User:Hannah C. Goldbach/Notebook/Biology 210 at AU: Difference between revisions
| (56 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Dr. Bentley's feedback == | == Dr. Bentley's feedback == | ||
< | =Effects of Beta-Blockers on Zebra Fish= | ||
==Purpose== | |||
Currently, Metoprolol is rated as a Risk Category C drug [http://www.mayoclinic.org/drugs-supplements/metoprolol-oral-route/before-using/drg-20071141], shortly meaning that there isn’t enough information to know whether or not the drug is harmful to human fetuses [https://chemm.nlm.nih.gov/pregnancycategories.htm]. As high blood pressure affects such a large portion of the adult American population, there is a great need for more information regarding the use of beta blockers such as metropolol, which slow heart rate, during pregnancy. This is the purpose of this experiment, to investigate the potential risks of using Metoprolol to decrease blood pressure during a pregnancy. | |||
==Methods & Materials == | |||
===Control=== | |||
As a control, 24 zebrafish embryos were started at the same time as the experimental, kept in individual wells with two mL solution of water and methylene blue, which acted as an anti-fungal. | |||
Every day they were measured, observed, fed 10μL of shrimp (?), and one mL of solution was replaced with fresh solution. The wells were kept covered at room temperature when they weren't being worked on. On days 8, 11, 14, and 15 controls were fixed for future reference. | |||
===Experimental=== | |||
The experimental were kept the same way as the control, except they were kept in two mL of a 50mg/L solution of metropolol. | |||
== Data & Observations == | |||
Data and observations maintained in a Google spreadsheet may be [https://docs.google.com/spreadsheets/d/1kMbv2FQr0OZ31zZ27pzXvfFnVU-tRuz8eJ3qNoz2stQ/edit?usp=sharing accessed here] | |||
Photos of the zebra fish throughout their development may be [https://drive.google.com/open?id=1yf_XxV5M76Z6u2olG1QW3a8j7sfF1rZkBg accessed here] | |||
==Conclusions & Future Directions == | |||
The experimental zebra fish had much slower heart rates, deformations, and died easier/faster than the control. | |||
=Transect= | |||
==February 25th, 2016== | |||
<h4> Week Seven: Vertebrates </h4> | |||
<h5> Purpose </h5> | |||
<p> Analyzing vertebrates present within the transect lends insight into trophic level interaction and the overall ecosystem present. </p> | |||
<h5> Methods and Materials </h5> | |||
<p> Throughout the overall experiment, the presence of different vertebral species was noted. Now, the collective observations are used to identify individual species as well as map out the food web and different trophic levels. </p> | |||
<h5> Data and Observations </h5> | |||
<p> The first week of the experiment, both deer droppings and nut meats were found, confirming the presence of deer. Later in the experiment squirrels were seen eating the nut meats, confirming the presence of squirrels within the transect. A dead rat was found in one of two rodent traps within the transect, and koi fish were observed in the pond. Although not always present, both robins and sparrows are surely present within the transect, as they are numerous not only on campus, but throughout Washington, D.C. | |||
<h5> Conclusions and Future Directions </h5> | |||
[[Image:DCHFoodWeb.jpg|right|400px|thumbnail|Figure 15: Transect Food Web]] | |||
The vertebrates found in the transect over the course of the experiment are all a part of the transect's ecological community - the collective species present which interact with each other regularly. These species range from Pseudomonas putida, the soil bacteria found, to Odocoileus virginianus, the white-tailed deer. These species can also be broken down into trophic levels: the algae, grasses, trees, and other plants are all primary producers; anything that feeds on the primary producers is considered a primary consumer; anything that feeds on a primary consumer is considered a secondary consumer, and so on. Most of the vertebrates found in transect four are herbivores, meaning they are mostly primary consumers. These trophic levels are also indicative of the carrying capacity, as each trophic level's carrying capacity depends on the population of the trophic level below it. The high levels of bacteria found in our samples provide more than adequate food resources for paramecium and small invertebrates - allowing those populations to thrive as well. The higher up in trophic levels a species sits, the smaller the carrying capacity will be as it will take more individuals of the previous level to support. | |||
White-Tailed Deer: Odocoileus virginianus [http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=180699] | |||
* Phylum: Chordata. | |||
* Class: Mammalia | |||
* Order: Artiodactyla | |||
* Family: Cervidae | |||
* Genus: Odocoileus | |||
* Specie: Odiocoileu virginianus | |||
The white-tailed deer are drawn to the transect because they most likely benefit from the grasses and water source within the transect. | |||
Grey Squirrel: Sciurus carolinensis [http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=180175] | |||
* Phylum: Chordata | |||
* Class: Mammalia | |||
* Order: Rodentia | |||
* Family: Sciuridae | |||
* Genus: Sciurus | |||
* Species: Sciurus carolinensis | |||
The grey squirrel benefits from the shelter offered within the transect, as well as the food (acorns) and water sources. | |||
Rat: Rattus rattus [http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=180362] | |||
* Phylum: Chordata | |||
* Class: Mammalia | |||
* Order: Rodentia | |||
* Family: Muridae | |||
* Genus: Rattus | |||
* Species: Rattus rattus | |||
Ever the opportunist, the rats present within the transect probably benefit more from the bordering dormitory, and what's within it, more than anything contained within the actual transect. While the acorns and seeds are inviting, surely the nearby garbage cans are more so. | |||
American Robin: Turdus migratorius [http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=179759] | |||
*Phylum: Chordata | |||
*Class: Aves | |||
*Order:Passeriformes | |||
*Family: Turdidae | |||
*Genus: Turdus | |||
*Species: Turdus migratorius | |||
The American Robin benefits from the trees and bushes, as shelter, the seeds and invertebrates, as a food source, and the pond as a water source. | |||
House Sparrow: Passer domesticus [http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=179628] | |||
*Phylum: Chordata | |||
*Class: Aves | |||
*Order: Passeriformes | |||
*Family: Passeridae | |||
*Genus: Passer | |||
*Species: Passer domesticus | |||
The House Sparrow also benefits from the trees and bushes, as shelter, the seeds and fruits or berries, as a food source, and the pond as a water source. | |||
--[[User:Hannah C. Goldbach|Hannah C. Goldbach]] 23:32, 16 March 2016 (EDT) | |||
==February 18th, 2016== | |||
<h4> Week Six: PCR Analysis</h4> | |||
[[Image:DCHgel.jpg|right|200px|thumbnail|Figure 12: PCR gel]] | |||
[[Image:TDCH.png|right|200px|thumbnail|Figure 13: Pseudomonas putida sequence (Colony 3)]] | |||
[[Image:GDCH.png|right|200px|thumbnail|Figure 14: Pseudomonas strain sequence (Colony 2)]] | |||
<h5> Purpose </h5> | |||
PCR analysis was done on two bacteria colonies cultured from the transect in order to identify them further than what could be accomplished using morphology. This allows for a more complete picture regarding the microbial life within the transect.</p> | |||
<h5> Methods and Materials </h5> | |||
<p>Two colonies were chosen earlier for sequencing based on morphological traits. Bacteria from each of the two colonies was placed in a PCR tube with 20μL of a primer/water mix. These PCR tubes were labeled and sent for sequencing at an outside facility. Once the sequences were returned, they were run through a BLAST analysis to identify the strain, or potential strain, of bacteria in the original colony.</p> | |||
<h5> Data and Observations </h5> | |||
<p>Through the BLAST analysis, the Colony 3 came back as a 100% match for Pseudomonas putida, and Colony 2 came back as a 45% match for some strain of Pseudomonas. </p> | |||
<h5> Conclusions and Future Directions </h5> | |||
<p> Pseudomonas putida is a strain of soil bacteria. Originally, we had noted that the bacteria were nonmotile, gram-negative staphylococci. Pseduomonas putida is gram-negative and motile, however the bacteria are actually rod-shaped [https://microbewiki.kenyon.edu/index.php/Pseudomonas_putida]. The strain of bright green colonies, as is Colony 2, were very small and difficult to locate or collect. This is most likely why the sequence came back as such a low match for anything, because there probably wasn't enough of the colony transferred to the PCR tube. In order to know for certain what Colony 2 is, the sequence would have to be run again. </p> | |||
*'''[[User:Hannah C. Goldbach|Hannah C. Goldbach]] 09:43, 1 March 2016 (EST)''': | |||
==February 11th, 2016== | |||
<h4>Week Five: Invertebrates</h4> | |||
[[Image:DCHBugs.png|right|400px|thumbnail|Figure 7: Invertebrate Samples]] | |||
<h5>Purpose</h5> | |||
<p> The purpose of this week's lab period was to identify invertebrates taken from a representative sample of leaf litter, and collected using a Berlese funnel. We expected to find few invertebrates given the current season and condition of our Transect. </p> | |||
<h5>Methods and Materials</h5> | |||
<p> A Berlese Funnel collection relies on the invertebrates instinctive dislike of light sources. A funnel of leaf litter, which drains into a 50% ethanol solution, is placed below a bright light and covered with foil. As the invertebrate seeks to climb away from the light source it crawls towards the bottom of the funnel, inevitably falling into the ethanol solution where the invertebrate becomes fixed. This method of collection allows a representative sample to be easily collected without needing to search the Transect for individual organisms. A week after the funnel was set up, the ethanol solution was removed from the bottom of the funnel, the collected invertebrates were drained into a dish and identified under a dissection scope. </p> | |||
<h5>Data and Observations</h5> | |||
<p>In the time allowed, we were able to identify five invertebrates. Figure 7 shows a centipede, identified by its segments and limbs. Figure 8 shows a millipede, identified also by its segmentation and numerous limbs. The weevil in figure 9 was identified by its snout and jointed antennae. Figure 11 shows a spider, identified by it's body segmentation and number of limbs. The Springtail in Figure 10 was identified by its long antennae, jointed limbs, and body segmentation. </p> | |||
<h5>Conclusions and Future Directions</h5> | |||
<p>The invertebrate shown in Figure 10 is a Springtail, also known as a "Snow Flea." These invertebrates earned this nickname because as the weather warms, they are known to crawl out of the snow. Our samples were collected shortly after a blizzard, which was followed by a period of warming. It makes sense that our transect, as well as many others, saw Springtails in the Berlese Funnel collections. It'd be interesting to repeat this experiment in the spring or summer, to see how the weather impacts the rise and fall of invertebrate populations. If the experiment were repeated, samples taken from underneath rocks or even the top layer of soil might produce more invertebrates. </p> | |||
--[[User:Hannah C. Goldbach|Hannah C. Goldbach]] 09:10, 24 February 2016 (EST) | |||
==February 4th, 2016== | |||
<h4>Week Four: Plants and Fungi</h4> | <h4>Week Four: Plants and Fungi</h4> | ||
[[Image:DCHPlants.png|right|500px|thumbnail|Figure 6: Plant Samples]] | |||
[[Image:DCHPlantObservation.png|right|500px|thumbnail|Table 3: Plant Characterization]] | |||
<h5>Purpose</h5> | <h5>Purpose</h5> | ||
<p>The purpose of this week's lab period was to identify and characterize plant life within the transect. Based on previous observations, we expected to find Red Oak trees, Hibiscus plants, and a variety of other flowering plants.</p> | |||
<h5>Materials and methods</h5> | <h5>Materials and methods</h5> | ||
<p> Using a pair of small scissors, samples of plant life were carefully trimmed, collected, and taken back to lab. There they were characterized and obvious physical features were observed. Some plant samples were put under closer observation. A hibiscus bud was cut into halves, so the internal structures could be observed. Vascularization, special structures, and mechanisms of reproduction were all observed and recorded.</p> | |||
<h5>Data and Observations</h5> | <h5>Data and Observations</h5> | ||
<p> The five collected plant samples, as seen in Figure 6, were brought back to the lab for analysis. Plant sample one was taken from a hibiscus plant, and was found to have intact flowers with complex internal reproductive structures and leaves with net-like veins. Plant sample two was taken from a patch of short grass near the stone walk way, and was found to have parallel veins. Plant sample three was taken from the ground near one of the benches. This sample had a small white flower with three inner petals, three outer petals, six stamen and a stigma, and leaves with parallel veins. Plant sample four was a small flower sprout, with parallel veins and flat leaves. Lastly, plant sample five was a section of ivy-looking leaves taken from a section of ground vines near the front of the transect. These leaves had net life veins, and because of their location near the ground under other bushes it was determined that the plant must also thrive in low levels of light. </p> | |||
<h5>Conclusions and Future directions</h5> | <h5>Conclusions and Future directions</h5> | ||
<p>All of our collected samples were found to be flowering plants, reproducing through stigma and stamen. Plant samples 1 and 5 were found to be dicots. This was concluded primarily based on their vein orientation, as many plants weren't flowering at this time. Plant samples 2, 3, and 4 were found to be monocots, based on their parallel vein layouts.</p> | |||
--[[User:Hannah C. Goldbach|Hannah C. Goldbach]] 16:57, 15 February 2016 (EST) | |||
== January 28th, 2016 == | == January 28th, 2016 == | ||
| Line 32: | Line 227: | ||
<h5>Conclusions and Future directions:</h5> | <h5>Conclusions and Future directions:</h5> | ||
<p>As can be seen in Table 1, there was far less growth in the nutrient agar plates with tetracycline. While bacterial growth was greatly inhibited, fungal growth was allowed to | <p>As can be seen in Table 1, there was far less growth in the nutrient agar plates with tetracycline. While bacterial growth was greatly inhibited, fungal growth was allowed to thrive. Tetracycline is an antibiotic which functions by inhibiting protein synthesis within the bacterial cell. As a broad-spectrum antibiotic, Tetracycline works on both gram-positive and gram-negative bacteria. However, because of it's availability and low side-effect rate, the antibiotic became a regular additive to cattle feed. The continual use of tetracycline to promote growth in meat-animals has led to a large resistance to the drug- it is now starting to work on continually fewer strains.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC99026/] | ||
As mentioned, four individual colonies were chosen for additional analysis. Based on the observations, it was determined that Colony 4 was not a bacterial colony, but a fungus. It was also very difficult to observe individual cells from Colony 1, so the only two remaining colonies were processed for PCR, which will be completed next lab period. </p> | As mentioned, four individual colonies were chosen for additional analysis. Based on the observations, it was determined that Colony 4 was not a bacterial colony, but a fungus. It was also very difficult to observe individual cells from Colony 1, so the only two remaining colonies were processed for PCR, which will be completed next lab period. </p> | ||
| Line 67: | Line 262: | ||
<p>The paramecium we found in our hay infusion produce energy by consuming other organisms, most likely the bacteria we also found, they contain DNA and reproduce asexually, and although they are unicellular they are capable of evolution; by these criteria the paramecium can be characterized as living. Given the duck pond, it was predicted that there would be more algae and protists then there were. However, it was later brought up that the lack of protists or algae could be because the layer of bacteria on top of the water was so thick that it suffocated anything living in the water. The water our sample was taken from was stagnant, which could cause the high bacteria count.</p> | <p>The paramecium we found in our hay infusion produce energy by consuming other organisms, most likely the bacteria we also found, they contain DNA and reproduce asexually, and although they are unicellular they are capable of evolution; by these criteria the paramecium can be characterized as living. Given the duck pond, it was predicted that there would be more algae and protists then there were. However, it was later brought up that the lack of protists or algae could be because the layer of bacteria on top of the water was so thick that it suffocated anything living in the water. The water our sample was taken from was stagnant, which could cause the high bacteria count.</p> | ||
<p>If the hay infusion were allowed to sit for another two months, the bacteria would probably continue to multiply until carrying capacity was reached. After that point, the bacteria population would remain fairly constant until all available resources in the hay infusion jar were consumed. The bacteria population would then start to die off.</p> | <p>If the hay infusion were allowed to sit for another two months, the bacteria would probably continue to multiply until carrying capacity was reached. After that point, the bacteria population would remain fairly constant until all available resources in the hay infusion jar were consumed. The bacteria population would then start to die off.</p> | ||
| Line 100: | Line 292: | ||
--[[User:Hannah C. Goldbach|Hannah C. Goldbach]] 17:12, 3 February 2016 (EST) | --[[User:Hannah C. Goldbach|Hannah C. Goldbach]] 17:12, 3 February 2016 (EST) | ||
Latest revision as of 13:32, 17 March 2016
Dr. Bentley's feedback
Effects of Beta-Blockers on Zebra Fish
Purpose
Currently, Metoprolol is rated as a Risk Category C drug [1], shortly meaning that there isn’t enough information to know whether or not the drug is harmful to human fetuses [2]. As high blood pressure affects such a large portion of the adult American population, there is a great need for more information regarding the use of beta blockers such as metropolol, which slow heart rate, during pregnancy. This is the purpose of this experiment, to investigate the potential risks of using Metoprolol to decrease blood pressure during a pregnancy.
Methods & Materials
Control
As a control, 24 zebrafish embryos were started at the same time as the experimental, kept in individual wells with two mL solution of water and methylene blue, which acted as an anti-fungal. Every day they were measured, observed, fed 10μL of shrimp (?), and one mL of solution was replaced with fresh solution. The wells were kept covered at room temperature when they weren't being worked on. On days 8, 11, 14, and 15 controls were fixed for future reference.
Experimental
The experimental were kept the same way as the control, except they were kept in two mL of a 50mg/L solution of metropolol.
Data & Observations
Data and observations maintained in a Google spreadsheet may be accessed here
Photos of the zebra fish throughout their development may be accessed here
Conclusions & Future Directions
The experimental zebra fish had much slower heart rates, deformations, and died easier/faster than the control.
Transect
February 25th, 2016
Week Seven: Vertebrates
Purpose
Analyzing vertebrates present within the transect lends insight into trophic level interaction and the overall ecosystem present.
Methods and Materials
Throughout the overall experiment, the presence of different vertebral species was noted. Now, the collective observations are used to identify individual species as well as map out the food web and different trophic levels.
Data and Observations
The first week of the experiment, both deer droppings and nut meats were found, confirming the presence of deer. Later in the experiment squirrels were seen eating the nut meats, confirming the presence of squirrels within the transect. A dead rat was found in one of two rodent traps within the transect, and koi fish were observed in the pond. Although not always present, both robins and sparrows are surely present within the transect, as they are numerous not only on campus, but throughout Washington, D.C.
Conclusions and Future Directions

The vertebrates found in the transect over the course of the experiment are all a part of the transect's ecological community - the collective species present which interact with each other regularly. These species range from Pseudomonas putida, the soil bacteria found, to Odocoileus virginianus, the white-tailed deer. These species can also be broken down into trophic levels: the algae, grasses, trees, and other plants are all primary producers; anything that feeds on the primary producers is considered a primary consumer; anything that feeds on a primary consumer is considered a secondary consumer, and so on. Most of the vertebrates found in transect four are herbivores, meaning they are mostly primary consumers. These trophic levels are also indicative of the carrying capacity, as each trophic level's carrying capacity depends on the population of the trophic level below it. The high levels of bacteria found in our samples provide more than adequate food resources for paramecium and small invertebrates - allowing those populations to thrive as well. The higher up in trophic levels a species sits, the smaller the carrying capacity will be as it will take more individuals of the previous level to support.
White-Tailed Deer: Odocoileus virginianus [3]
- Phylum: Chordata.
- Class: Mammalia
- Order: Artiodactyla
- Family: Cervidae
- Genus: Odocoileus
- Specie: Odiocoileu virginianus
The white-tailed deer are drawn to the transect because they most likely benefit from the grasses and water source within the transect.
Grey Squirrel: Sciurus carolinensis [4]
- Phylum: Chordata
- Class: Mammalia
- Order: Rodentia
- Family: Sciuridae
- Genus: Sciurus
- Species: Sciurus carolinensis
The grey squirrel benefits from the shelter offered within the transect, as well as the food (acorns) and water sources.
Rat: Rattus rattus [5]
- Phylum: Chordata
- Class: Mammalia
- Order: Rodentia
- Family: Muridae
- Genus: Rattus
- Species: Rattus rattus
Ever the opportunist, the rats present within the transect probably benefit more from the bordering dormitory, and what's within it, more than anything contained within the actual transect. While the acorns and seeds are inviting, surely the nearby garbage cans are more so.
American Robin: Turdus migratorius [6]
- Phylum: Chordata
- Class: Aves
- Order:Passeriformes
- Family: Turdidae
- Genus: Turdus
- Species: Turdus migratorius
The American Robin benefits from the trees and bushes, as shelter, the seeds and invertebrates, as a food source, and the pond as a water source.
House Sparrow: Passer domesticus [7]
- Phylum: Chordata
- Class: Aves
- Order: Passeriformes
- Family: Passeridae
- Genus: Passer
- Species: Passer domesticus
The House Sparrow also benefits from the trees and bushes, as shelter, the seeds and fruits or berries, as a food source, and the pond as a water source.
--Hannah C. Goldbach 23:32, 16 March 2016 (EDT)
February 18th, 2016
Week Six: PCR Analysis
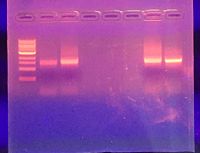


Purpose
PCR analysis was done on two bacteria colonies cultured from the transect in order to identify them further than what could be accomplished using morphology. This allows for a more complete picture regarding the microbial life within the transect.
Methods and Materials
Two colonies were chosen earlier for sequencing based on morphological traits. Bacteria from each of the two colonies was placed in a PCR tube with 20μL of a primer/water mix. These PCR tubes were labeled and sent for sequencing at an outside facility. Once the sequences were returned, they were run through a BLAST analysis to identify the strain, or potential strain, of bacteria in the original colony.
Data and Observations
Through the BLAST analysis, the Colony 3 came back as a 100% match for Pseudomonas putida, and Colony 2 came back as a 45% match for some strain of Pseudomonas.
Conclusions and Future Directions
Pseudomonas putida is a strain of soil bacteria. Originally, we had noted that the bacteria were nonmotile, gram-negative staphylococci. Pseduomonas putida is gram-negative and motile, however the bacteria are actually rod-shaped [8]. The strain of bright green colonies, as is Colony 2, were very small and difficult to locate or collect. This is most likely why the sequence came back as such a low match for anything, because there probably wasn't enough of the colony transferred to the PCR tube. In order to know for certain what Colony 2 is, the sequence would have to be run again.
- Hannah C. Goldbach 09:43, 1 March 2016 (EST):
February 11th, 2016
Week Five: Invertebrates

Purpose
The purpose of this week's lab period was to identify invertebrates taken from a representative sample of leaf litter, and collected using a Berlese funnel. We expected to find few invertebrates given the current season and condition of our Transect.
Methods and Materials
A Berlese Funnel collection relies on the invertebrates instinctive dislike of light sources. A funnel of leaf litter, which drains into a 50% ethanol solution, is placed below a bright light and covered with foil. As the invertebrate seeks to climb away from the light source it crawls towards the bottom of the funnel, inevitably falling into the ethanol solution where the invertebrate becomes fixed. This method of collection allows a representative sample to be easily collected without needing to search the Transect for individual organisms. A week after the funnel was set up, the ethanol solution was removed from the bottom of the funnel, the collected invertebrates were drained into a dish and identified under a dissection scope.
Data and Observations
In the time allowed, we were able to identify five invertebrates. Figure 7 shows a centipede, identified by its segments and limbs. Figure 8 shows a millipede, identified also by its segmentation and numerous limbs. The weevil in figure 9 was identified by its snout and jointed antennae. Figure 11 shows a spider, identified by it's body segmentation and number of limbs. The Springtail in Figure 10 was identified by its long antennae, jointed limbs, and body segmentation.
Conclusions and Future Directions
The invertebrate shown in Figure 10 is a Springtail, also known as a "Snow Flea." These invertebrates earned this nickname because as the weather warms, they are known to crawl out of the snow. Our samples were collected shortly after a blizzard, which was followed by a period of warming. It makes sense that our transect, as well as many others, saw Springtails in the Berlese Funnel collections. It'd be interesting to repeat this experiment in the spring or summer, to see how the weather impacts the rise and fall of invertebrate populations. If the experiment were repeated, samples taken from underneath rocks or even the top layer of soil might produce more invertebrates.
--Hannah C. Goldbach 09:10, 24 February 2016 (EST)
February 4th, 2016
Week Four: Plants and Fungi

Purpose
The purpose of this week's lab period was to identify and characterize plant life within the transect. Based on previous observations, we expected to find Red Oak trees, Hibiscus plants, and a variety of other flowering plants.
Materials and methods
Using a pair of small scissors, samples of plant life were carefully trimmed, collected, and taken back to lab. There they were characterized and obvious physical features were observed. Some plant samples were put under closer observation. A hibiscus bud was cut into halves, so the internal structures could be observed. Vascularization, special structures, and mechanisms of reproduction were all observed and recorded.
Data and Observations
The five collected plant samples, as seen in Figure 6, were brought back to the lab for analysis. Plant sample one was taken from a hibiscus plant, and was found to have intact flowers with complex internal reproductive structures and leaves with net-like veins. Plant sample two was taken from a patch of short grass near the stone walk way, and was found to have parallel veins. Plant sample three was taken from the ground near one of the benches. This sample had a small white flower with three inner petals, three outer petals, six stamen and a stigma, and leaves with parallel veins. Plant sample four was a small flower sprout, with parallel veins and flat leaves. Lastly, plant sample five was a section of ivy-looking leaves taken from a section of ground vines near the front of the transect. These leaves had net life veins, and because of their location near the ground under other bushes it was determined that the plant must also thrive in low levels of light.
Conclusions and Future directions
All of our collected samples were found to be flowering plants, reproducing through stigma and stamen. Plant samples 1 and 5 were found to be dicots. This was concluded primarily based on their vein orientation, as many plants weren't flowering at this time. Plant samples 2, 3, and 4 were found to be monocots, based on their parallel vein layouts.
--Hannah C. Goldbach 16:57, 15 February 2016 (EST)
January 28th, 2016
Week Three: Microbiology




Purpose:
The purpose of this week's lab was to observe what, if any, populations of bacteria are present in the representative sample from Transect 4. It's predicted that incubated agar plates, which were created a week prior using samples from the hay infusion culture, will have large quantities of bacteria present given the location of Transect 4 near the duck pond and given previous observations.
Materials and methods
A week prior to this lab period, two identical serial dilutions were created. The initial dilution for both sets was made with 100μL of water from the hay infusion culture, and 10mLs of broth. From there, 100μL were transferred from each dilution to the next. The product was a set of dilutions with concentrations 10-2, 10-4, 10-6, and 10-8. Two sets of nutrient agar plates, one with tetracycline and one without, were both inoculated with 100μL of of broth for final concentrations of 10-3, 10-5, 10-7, and 10-9. These inoculated plates were allowed to incubate at room temperature for one week before observations were made. Upon returning to the incubated plates, colonies were counted and observed under a dissection microscope. Four individual colonies were chosen to undergo a Gram stain procedure which would indicate the presence of peptidoglycan in the bacteria's cell walls. Using a sterile loop, a small amount of bacterial growth was transferred onto a slide and then heat-fixed. The smear was covered with crystal violet for one minutes, rinsed, covered with Gram's iodine mordant for one minutes, rinsed, covered with 95% alcohol for 10-20 seconds, rinsed, covered with safranin stain for 20-30 seconds, and rinsed a final time before being allowed to dry. The dry slide was then observed under a microscope at 40x - a pink color indicated a gram-negative bacteria while a blue-purple color indicated a gram-positive bacteria. A wet-mount of each colony was then made so that cell size, motility, and shape could be observed. Based on the observations of the wet-mounts and the results of the gram-stain, two colonies were chosen to go through PCR for 16S amplification. The PCR tubes were prepared with 20μL of primer/water and a very small amount of bacterial growth. They were then placed in the PCR machine so that next lab period the products may be run on an agarose gel.
Data and Observations:
The hay infusion culture appeared much as it did last week; the smell was the same and there was still a heavy film on top of the water. The water appeared clearer and the layer of sediment on the bottom was thicker. The hay infusion culture was discarded after this lab. The results of the serial dilutions may be found in Table 1. From the thousands of colonies that grew, four main types of colonies were identified (Figure 4). Colony 1 was a medium sized, smooth, round, orange colony, colony 2 was a very small, smooth, round, green colony, colony 3 was a medium sized, smooth, round, tan colony, and colony 4 was a large, filamentous, white colony. These four colonies were stained and observed. The results of both the gram stain and the wet-mount may be found in Table 2. Colony 4 contained very large, highly motile, oddly-shaped cells.
Conclusions and Future directions:
As can be seen in Table 1, there was far less growth in the nutrient agar plates with tetracycline. While bacterial growth was greatly inhibited, fungal growth was allowed to thrive. Tetracycline is an antibiotic which functions by inhibiting protein synthesis within the bacterial cell. As a broad-spectrum antibiotic, Tetracycline works on both gram-positive and gram-negative bacteria. However, because of it's availability and low side-effect rate, the antibiotic became a regular additive to cattle feed. The continual use of tetracycline to promote growth in meat-animals has led to a large resistance to the drug- it is now starting to work on continually fewer strains.[9] As mentioned, four individual colonies were chosen for additional analysis. Based on the observations, it was determined that Colony 4 was not a bacterial colony, but a fungus. It was also very difficult to observe individual cells from Colony 1, so the only two remaining colonies were processed for PCR, which will be completed next lab period.
--Hannah C. Goldbach 23:11, 9 February 2016 (EST)
January 21st, 2016
Week Two: Algae and Protists


Purpose
The purpose of this lab was to observe and record what, if any, algae and protists were present in the hay infusion made last lab period. It's predicted, given the location and features of Transect 4 as well as the physical appearance of the hay infusion, that both algae and protists will be present in the samples taken from the hay infusion.
Materials and Methods
The hay infusion used for this lab was created using 12g of organic matter from Transect 4, 500mLs of Deerpark water, and 0.1g dried milk. The components were mixed and allowed to settle in an open jar for a week prior to this lab period. At the start of this lab period, the obvious physical characteristics were observed and recorded before wet mounts were made from three different niches in the hay infusion for microscopic observation. Observations made from these wet mounts were used in conjunction with a dichotomous key to identify what, if any, algae and protists were present in the hay infusion, and therefore also present in Transect 4.
Data and Observations
Upon returning to our hay infusion this week, we found that the sediment had settled to the bottom of the jar, the water had turned cloudy, and a thick layer of gray scum had formed on top of the water. Other than the layer of scum, which is believed to be bacteria, there was no other apparent life. We took three samples, one from the top layer of scum, one from the water in the middle of the jar, and one from the sediment on the bottom. The top layer of scum was all bacteria, there was no other evidence of protists or algae. The sample from the bottom layer of sediment produced little to no protists or algae as well. We were able to find paramecium in the water from the middle of the jar, as well as more bacteria.
The paramecium we were able to identify were clear in color and roughly oval shaped with visible organelles, cilia, and oral grooves. The bacteria from the top layer were so numerous that looking at the prepared slide was like watching static – a solid wall of tiny, tiny vibrating dots.
Conclusions and Future Directions
The paramecium we found in our hay infusion produce energy by consuming other organisms, most likely the bacteria we also found, they contain DNA and reproduce asexually, and although they are unicellular they are capable of evolution; by these criteria the paramecium can be characterized as living. Given the duck pond, it was predicted that there would be more algae and protists then there were. However, it was later brought up that the lack of protists or algae could be because the layer of bacteria on top of the water was so thick that it suffocated anything living in the water. The water our sample was taken from was stagnant, which could cause the high bacteria count.
If the hay infusion were allowed to sit for another two months, the bacteria would probably continue to multiply until carrying capacity was reached. After that point, the bacteria population would remain fairly constant until all available resources in the hay infusion jar were consumed. The bacteria population would then start to die off.
--Hannah C. Goldbach 17:12, 3 February 2016 (EST)
January 14th, 2016
Week One: Transect Description
Purpose
The purpose of this week's lab was to observe and record obvious physical characteristics of a given plot of land on American University's campus. These observations will then be used to make more detailed hypotheses and conclusions about the transect in relation to the rest of Campus life. American University is an urban campus, and it is expected that the effects of daily city activity will be evident in the recordings and samples taken from even a small plot of land like Transect 4 (Roper Hall Duck Pond).

Materials and Methods
The lab group visited the transect and recorded all evident abiotic and biotic components and other significant features were noted. In addition to these lists, photographs were taken and an aerial map was drawn to further document the transect's features. Samples of soil and plant matter were also collected for creating a hay infusion.
Data and Observations
Transect 4 is a plot of land located in the South-East corner of campus. Bordered by Roper Hall on two sides, the transect features a storm drain, a stone walkway, and a duck pond, which is covered with netting at this time of year. As a National Wildlife Federation Certified Wildlife Habitat, the transect also provides cover for wildlife in the forms of tall grasses, seven bushes, and two trees. The transect probably receives the most sunlight in the early to mid morning, after then the sun is blocked by Roper Hall. Since the transect is located next to a dorm building, students often cross through using the stone walkway - a few pieces of garbage were also scattered here and there.
- Abiotic Features: Frame over the pond, big rocks (5), stepping stones (51), medium pond rocks (39), little rocks in and around the pond (70-100), pond water - partially ice over, duck sculptures, fake rock, storm drain, rodent traps, signage, benches (3), sprinkler, gas/water pipes, wrappers (3)
- Biotic Features: People (4), squirrels (3), hibiscus plants (4), leafless bushes (2), Dogwood tree, Oak tree, acorns, dead plants, leaves, budding bulbs, dead rat
Conclusions and future directions
The physical characteristics were documented and observations were recorded. Signs of daily city activity were evident, as the transect received heavy foot traffic and a fair amount of pollution. Organic matter was collected to create a hay infusion, which will lend more insight in next week's lab period.
--Hannah C. Goldbach 17:12, 3 February 2016 (EST)