User:Jake Mulroy/Notebook/Biology 210 at AU: Difference between revisions
Jake Mulroy (talk | contribs) No edit summary |
Jake Mulroy (talk | contribs) No edit summary |
||
| Line 51: | Line 51: | ||
'''2/2/2016''' | '''2/2/2016''' | ||
Upon observing our Hay Infusion after another week had passed, we noticed there was less water in it. This was most likely due to evaporation. Another observation was that the smell was actually better. This was surprising as I would have expected it to smell worse. However, maybe it smelled better because something that had died finally had aired out in the open jar. There was also more scum and the water was dirtier, this could be due to the dirt that was inside breaking down. Archaea species will not have grown on the plates because it is not an extreme conditions. Archaea usually grow in hot springs or salt late, not agar plates. | Purpose: To discover the amount of bacteria in our hay infusion and how to morphological categorize these bacterium. Also, to test the antibiotic resistance in these bacterium and to use PCR and DNA sequencing and confirm our bacterial findings. | ||
Materials and Methods: | |||
Count the number of bacteria that grew on the eight agar plates. Record findings in table 1.Collect four samples, two from the nutreint agar plates and two from the nutrient agar plates with tet. Make a wet mount and study the slides under the miscroscope. After categorizing the bacteria, proceed to gram taining them. After gram staining them, look at them under the miscrosope again. Record results in table 2. Finally, set up PCR 16s amplification. | |||
Data and Conclusions: | |||
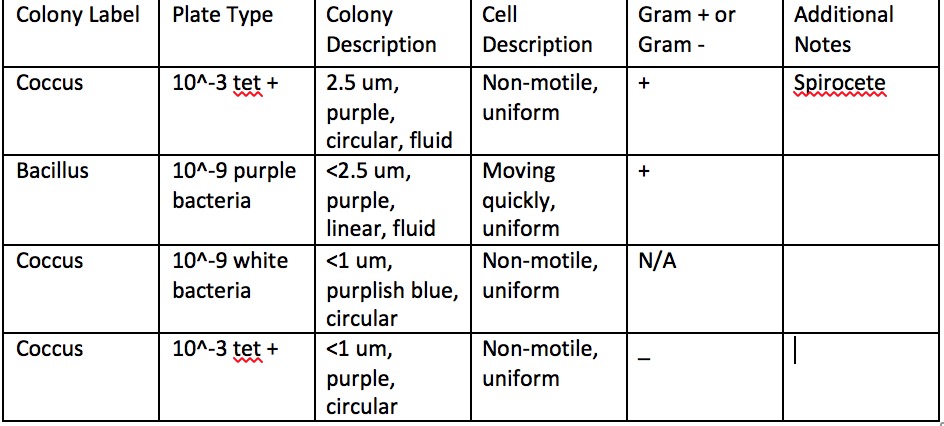
Upon observing our Hay Infusion after another week had passed, we noticed there was less water in it. This was most likely due to evaporation. Another observation was that the smell was actually better. This was surprising as I would have expected it to smell worse. However, maybe it smelled better because something that had died finally had aired out in the open jar. There was also more scum and the water was dirtier, this could be due to the dirt that was inside breaking down. Archaea species will not have grown on the plates because it is not an extreme conditions. Archaea usually grow in hot springs or salt late, not agar plates. According to our results, it seems that the agar plates without tetracycline experienced far more bacterial growth than the plates with tetracycline. this could mean that tetracycline is an efficient antibiotic that inhibits bacteria growth. However, there was one bacteria on the 10^-3 tet + that had bacterial growth. This could mean that that bacteria was resistant to the antibiotic. | |||
During this part of the lab, we ran into some difficulty. We could not identify bacteria species on the microscope with the help of the TA and UTA. It was very hard to characterize the bacteria we did find. It was even harder to accomplish the gram staining. Only a few bacteria slides were successfully gram stained. On the table, one colony could not be successfully gram stained. Because of our struggles, we could not get entirely accurate results. | |||
Table 1: 100-fold Serial Dilutions Results | Table 1: 100-fold Serial Dilutions Results | ||
[[Image:Screen Shot 2016-02-02 at 5.15.32 PM.jpg]] | [[Image:Screen Shot 2016-02-02 at 5.15.32 PM.jpg]] | ||
Table 2: Bacteria Characterization | Table 2: Bacteria Characterization | ||
[[Image:Screen Shot 2016-02-02 at 5.31.06 PM.jpg]] | [[Image:Screen Shot 2016-02-02 at 5.31.06 PM.jpg]] | ||
[[Image:IMG 1214.JPG]] | [[Image:IMG 1214.JPG]] | ||
Revision as of 14:47, 5 February 2016
1/26/2016
Abiotic Factors
Five wooden benches, Two metal signs, A drainage system, A stone path, A tiny pond, A bird feeder, Animal tracks, Snow, Roper building, Trash cans, Artificial light, Rocks
Biotic Factors
Oak tree, Persian Parrotia, Bushes, Shrubs, Organisms living in water, Organisms living in dirt, Leaves, Grass, Squirrels, Birds, Human interaction
General Description
When walking towards transect four, it is obvious to realize that the area is a designated green-space for Roper Hall. The area is flanked by Roper Hall on one side and by trees and shrubs on the other. There is a stone path that was placed in the middle of the transect in order to make it easily walkable. Five worn wooden benches flank the wooden path in order to give people a place to sit and admire the green-space. Towards the far left of the transect lies a tiny pond that is fenced off. The pond has a small marble statue and sign that states “Certified Wildlife Habitat.” During the day, animal tracks can be seen as well as squirrels and birds who use the large oak tree as shelter. The ground crunches under your feet due to the fallen leaves and winter crust. Small pieces of grass dot the ground as do rocks and leaves. The shrubs and bushes create a barrier around the transect in order to shelter the wildlife found within. After the snow had fallen, the area became extremely quiet. The snow remained untouched and the area was shunned by humans. Only the sound of melting snow and squirrels scurrying up the oak tree could be heard and seen. J.M.
1/26/2016
Hay Infusion Lab
Purpose: The purpose of this lab was to be able to use a dichotomous key to identify unknown protists in our hay infusion. Another purpose was to identify the characteristics of algae and protists. Finally, we were to identify and record the algae and protists within our transect.
Materials and Methods: The first thing to do in this lab was to take a sample from the hay infusion and look at it under a microscope. Using the dichotomous key, the algae and protist present were to be identified and drawn. Samples from different niches within the hay infusion had to be tested it order to see which kind of life they contained.
Figure 1 is the surface niche at 10x magnification. It shows paramecium that range in size from 10um to 100um. Figure 2 also shows the paramecium in the surface niche but this time the paramecium are sized 200um.
Figure 3 is also the surface niche at 10x magnification but this shows a vorticella organism sized 30um. Figure 4 is the bottom niche at 10x magnification but this just showed paramecium of sizes 50-100um.
Figure 5 depicts the niche that was located near an acorn at 10x magnification. Here, paramecium multimicronueleatum existed and ranged from sizes 25-250um.
Conclusion: After locating our hay infusion, the color as a dark, murky brown. There was obvious scum on the side and a brown film on the surface of the water. The bottom was also more clear than the surface of the hay infusion. There was no apparent life located within the hay infusion. After opening the jar, the smell was repulsive. It smelled similar to rotten eggs and the top had to be put back on to avoid the pungent odor. The three organisms found within the hay infusion were paramecium, paramecium multimicronueleatum, and vorticella. All three were motile and they ranged in size from 25-250um. Paramecium use energy to move and they get this energy from feeding on microorganisms like bacteria and algae. Paramecium reproduce both sexually and asexually. They have DNA and continue to evolve to this day. If the hay infusion were to continue to grow for another month i predict that it would eventually hold no life. There would be no food because nothing is growing inside of it. Lack of food and sunlight would lead the community to die within two months.
Heres a photo of the serial dilutions we set up for next weeks lab.
J.M.
2/2/2016 Purpose: To discover the amount of bacteria in our hay infusion and how to morphological categorize these bacterium. Also, to test the antibiotic resistance in these bacterium and to use PCR and DNA sequencing and confirm our bacterial findings.
Materials and Methods: Count the number of bacteria that grew on the eight agar plates. Record findings in table 1.Collect four samples, two from the nutreint agar plates and two from the nutrient agar plates with tet. Make a wet mount and study the slides under the miscroscope. After categorizing the bacteria, proceed to gram taining them. After gram staining them, look at them under the miscrosope again. Record results in table 2. Finally, set up PCR 16s amplification.
Data and Conclusions:
Upon observing our Hay Infusion after another week had passed, we noticed there was less water in it. This was most likely due to evaporation. Another observation was that the smell was actually better. This was surprising as I would have expected it to smell worse. However, maybe it smelled better because something that had died finally had aired out in the open jar. There was also more scum and the water was dirtier, this could be due to the dirt that was inside breaking down. Archaea species will not have grown on the plates because it is not an extreme conditions. Archaea usually grow in hot springs or salt late, not agar plates. According to our results, it seems that the agar plates without tetracycline experienced far more bacterial growth than the plates with tetracycline. this could mean that tetracycline is an efficient antibiotic that inhibits bacteria growth. However, there was one bacteria on the 10^-3 tet + that had bacterial growth. This could mean that that bacteria was resistant to the antibiotic. During this part of the lab, we ran into some difficulty. We could not identify bacteria species on the microscope with the help of the TA and UTA. It was very hard to characterize the bacteria we did find. It was even harder to accomplish the gram staining. Only a few bacteria slides were successfully gram stained. On the table, one colony could not be successfully gram stained. Because of our struggles, we could not get entirely accurate results.
Table 1: 100-fold Serial Dilutions Results

Table 2: Bacteria Characterization
Because we had such difficulty finding the bacteria, we didn't have a lot of time to draw the bacteria under the microscope. Here are four pictures that show the bacteria we did find under the microscope.
Jake Mulroy







