User:James C. Schwabacher/Notebook/CHEM-571/2013/09/04
 Biomaterials Design Lab Biomaterials Design Lab
|
<html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> |
|
Objective
Procedure
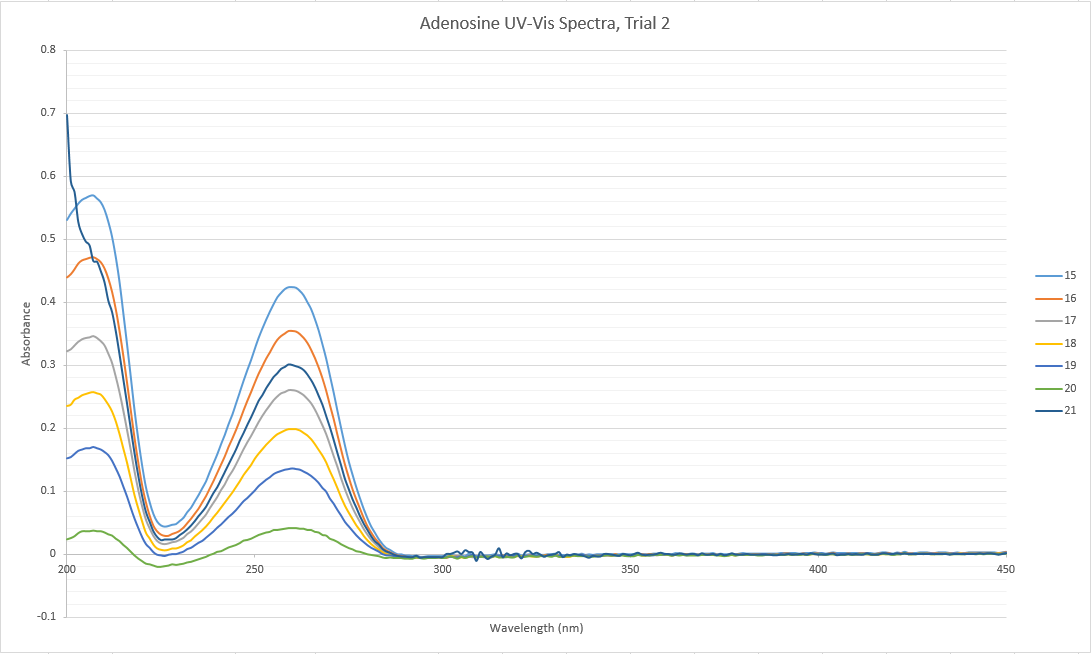
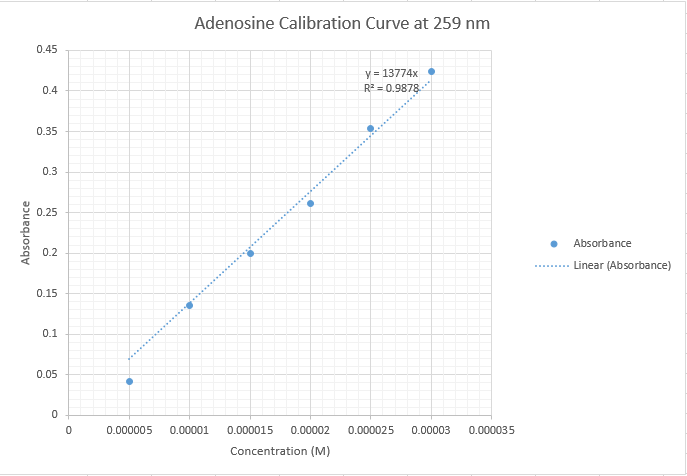
DataMatt Hartings Why does some of this data go below zero? You should re-correct this. The baseline should be zero for this one. NotesAll of the data collected yesterday was found to be systematically lower than the rest of the class' data. Thus, the data from the inosine trial was not included in the overall group data. Furthermore, a trial 2 of adenosine dilutions was run, using a new stock solution. This second set of data fit with the overall group data, and our first trial was discarded. It is most probable that there was an error in making the first set of stock solutions yesterday that resulted in the incomplete transfer of the massed solids into the 100 mL volumetric flasks. Having a smaller amount of solute in the solvent than calculated would result in systematically lower absorbance results.
| |