User:Javier Vinals Camallonga/Notebook/Javier Vinals notebook/2014/04/08: Difference between revisions
From OpenWetWare
(Autocreate 2014/04/08 Entry for User:Javier_Vinals_Camallonga/Notebook/Javier_Vinals_notebook) |
(fix raw html notebook nav) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
|- | |- | ||
|style="background-color: #EEE"|[[Image:owwnotebook_icon.png|128px]]<span style="font-size:22px;"> Project name</span> | |style="background-color: #EEE"|[[Image:owwnotebook_icon.png|128px]]<span style="font-size:22px;"> Project name</span> | ||
|style="background-color: #F2F2F2" align="center"| | |style="background-color: #F2F2F2" align="center"|[[File:Report.png|frameless|link={{#sub:{{FULLPAGENAME}}|0|-11}}]][[{{#sub:{{FULLPAGENAME}}|0|-11}}|Main project page]]<br />{{#if:{{#lnpreventry:{{FULLPAGENAME}}}}|[[File:Resultset_previous.png|frameless|link={{#lnpreventry:{{FULLPAGENAME}}}}]][[{{#lnpreventry:{{FULLPAGENAME}}}}{{!}}Previous entry]] }}{{#if:{{#lnnextentry:{{FULLPAGENAME}}}}|[[{{#lnnextentry:{{FULLPAGENAME}}}}{{!}}Next entry]][[File:Resultset_next.png|frameless|link={{#lnnextentry:{{FULLPAGENAME}}}}]]}} | ||
|- | |- | ||
| colspan="2"| | | colspan="2"| | ||
| Line 10: | Line 10: | ||
==Objective== | ==Objective== | ||
* Take conductivity measurements on room temperature solutions from 3/26/14. | |||
* Run UV-Vis and AA on solutions at 4°C from 3/26/14. | |||
==Procedure== | |||
===Conductivity Measurement of Pure Variables at Room Temperature=== | |||
[[Image:4.8.conductivity.room.png|800px|]] | |||
===Atomic Absorption Preparation=== | |||
'''Creating the Gold Stock Solutions''' | |||
# Add 50 μL of HAuCl<sub>4</sub>·3H<sub>2</sub>O and 4950 μL of distilled water to a Falcon tube, for a final concentration of 10 μg/mL Au. | |||
# Add 100 μL of HAuCl<sub>4</sub>·3H<sub>2</sub>O and 4900 μL of distilled water to a Falcon tube, for a final concentration of 20 μg/mL Au. | |||
# Add 150 μL of HAuCl<sub>4</sub>·3H<sub>2</sub>O and 4850 μL of distilled water to a Falcon tube, for a final concentration of 30 μg/mL Au. | |||
# Add 200 μL of HAuCl<sub>4</sub>·3H<sub>2</sub>O and 4800 μL of distilled water to a Falcon tube, for a final concentration of 40 μg/mL Au. | |||
# Add 250 μL of HAuCl<sub>4</sub>·3H<sub>2</sub>O and 4750 μL of distilled water to a Falcon tube, for a final concentration of 50 μg/mL Au. | |||
'''Atomic Absorption Samples''' | |||
Solutions with the following Au:lysozyme ratio at 4°C were run on the AA: | |||
* 30:1 lysozyme-AuNP with 0.03 M MgCl<sub>2</sub>, CaCl<sub>2</sub>, NaCl, KCl, MES, citric acid (0.0002316 M 2,2 bipyridine) | |||
* 30:1 lysozyme-AuNP with 0.06 M MgCl<sub>2</sub>, CaCl<sub>2</sub>, NaCl, KCl, MES, citric acid (0.0004544 M 2,2 bipyridine) | |||
* 30:1 lysozyme-AuNP with 0.09 M MgCl<sub>2</sub>, CaCl<sub>2</sub>, NaCl, KCl, MES, citric acid (0.0006772 M 2,2 bipyridine) | |||
==Figures== | |||
===Atomic Absorption=== | |||
[[Image:4.8.aa.all.png|700px|]] | |||
[[Image:4.8.2.2.bipy.png|700px|]] | |||
===UV-Vis=== | |||
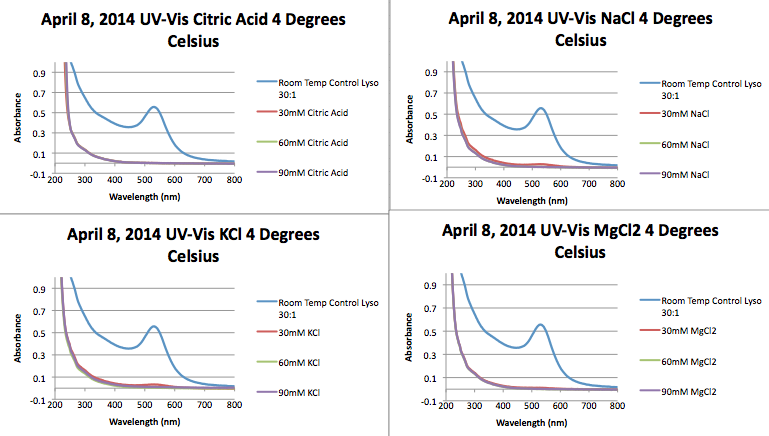
*The following UV-Vis are for 30:1 lysozyme-AuNP samples made on March 26, 2014. The samples were synthesized using the method we've been using all year and variables were added added synthesis. The samples were then cooled to 4°C to see the effect on fiber formation. | |||
* Note that all for all samples with the exception of the 2,2 bipyridine samples caused almost all AuNPs to fall out of solution. | |||
[[Image:Screen_Shot_2014-04-09_at_1.29.09_PM.png]] | |||
[[Image:Screen_Shot_2014-04-09_at_1.29.34_PM.png]] | |||
[[Image:Screen_Shot_2014-04-09_at_1.29.45_PM.png]] | |||
*Note that due to the measure of accuracy of the equipment used, the percentages that are close in value might not different enough to be considered significant. This is because the values used to calculate these percentages differed by one decimal point ex: .0005 and .0004 | |||
[[Image:Screen_Shot_2014-04-09_at_1.53.20_PM.png]] | |||
Latest revision as of 23:53, 26 September 2017
Entry title
Objective
ProcedureConductivity Measurement of Pure Variables at Room TemperatureAtomic Absorption PreparationCreating the Gold Stock Solutions
Atomic Absorption Samples Solutions with the following Au:lysozyme ratio at 4°C were run on the AA:
FiguresAtomic Absorption
UV-Vis
| |