User:Jihwan Lee/Notebook/Mini-prep: Ethanol V.S. WN Buffer/Entry Base: Difference between revisions
Jihwan Lee (talk | contribs) (Autocreated Lab Notebook name=User:Jihwan Lee/Notebook/Mini-prep: Ethanol V.S. WN Buffer/Entry Base, content from MediaWiki:EntryContentDefault) |
Jihwan Lee (talk | contribs) No edit summary |
||
| Line 7: | Line 7: | ||
<!-- ##### DO NOT edit above this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit above this line unless you know what you are doing. ##### --> | ||
==Entry title== | ==Entry title== | ||
==INTRODUCTION== | |||
Our investigation involved mini-preparation, which is a rapid, small-scale extraction of plasmid from bacteria. Viogene kit, which is used for this process contains a solution called WN buffer. The main purpose of WN buffer is to remove protein and degraded RNA residues from the membrane. It consists of 75% to 80% ethanol and chaotropic salts, which enhances the removal of degraded RNA residue. The main objective of our investigation was to use this viogene kit to do plasmid extraction and in the process observe whether WN buffer or 75% ethanol gives higher DNA yield and purity. Yield was determined by the concentration of DNA (ng/ml) and purity of DNA was determined by measuring A260/A280 and A260/230 ratio and observing how far this ratio deviates from the optimal purity ratio. DNA absorbs at both 260nm and 280nm whereas protein only absorbs at 280nm. So, a pure sample of DNA will have A260/A280 ratio around 1.8 and below 1.8 identifies possible protein contamination. Similarly organic contaminants such as phenol absorb light of 230nm wavelength whereas nucleic acids absorb at 260nm. So A260/A230 ratio can also tell us about the purity level of plasmid DNA. | |||
==OBJECTIVES== | |||
Compare the quality and the yield of the plasmid DNA extracted and washed using 75% ethanol and WN buffer from the Viogene kit. | |||
==EXPERIMENT DESIGN== | |||
We used three different samples to clean the extracted plasmid DNA: water, 75% ethanol and WN buffer provided by the Viogene kit. Water served as a negative control and WN buffer served as a positive control. 75% ethanol was tested to see how much yield and purity of DNA it gives and we compared this with the yield and purity of DNA WN buffer gives. With each person of our group carrying out the full experiment, we obtained 4 sets of data for yield and purity of DNA extract | |||
==RESULTS== | |||
'''NanoDrop Results - Yield, A280/A260 an A260/A230 ratio of plasmid DNA''' | |||
The average concentration of plasmid DNA sample washed using water during the wash step was lowest with 6.5ng/ml and with standard deviation of 2.8. The average concentration of plasmid DNA washed using WN buffer was around five times that of water with 34.5ng/ml. The standard deviation, however, was also around fives times bigger with 10.4. The average concentration of plasmid DNA washed using 75% ethanol was highest with 46.65ng/ml and standard deviation of 12.9. The error bars for ethanol and WN buffer sample overlap. | |||
[[Image:Average_Yield_of_DNA.png]] | |||
The purity level of plasmid DNA was represented by A260/A280 ratio. Average A260/A280 ratio of plasmid DNA sample washed using water was highest with 2.09 and with standard deviation 0.25. The average A260/A80 ratio of plasmid DNA washed using WN buffer was lower than that of water with 1.93. The standard deviation was relatively small with 0.027. The average A260/A80 ratio of plasmid DNA washed using 75% ethanol was lowest with 1.89 and standard deviation of 0.042. Water sample has much higher average A260/A80 ratio, than both ethanol and WN buffer sample. However, the average A260/A80 ratio between ethanol and WN buffer were similar and differed by less than 0.05. | |||
[[Image:Average_260-230_ratio_.png]] | |||
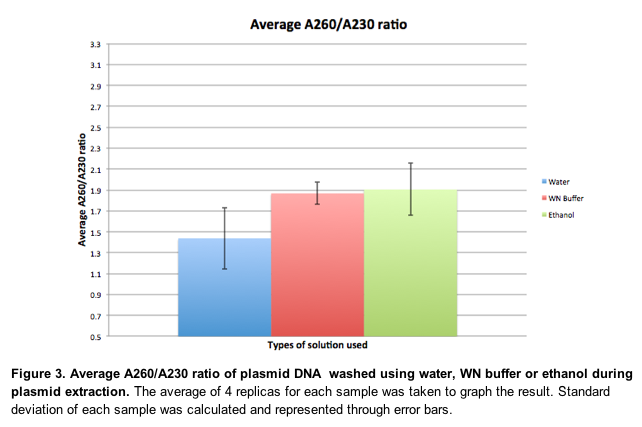
A260/A230 ratio is another way of testing the purity level of plasmid DNA. The average A260/A230 ratio of plasmid DNA washed using water was lowest with 1.44. Standard deviation was 0.29. The average A260/A230 ratio of plasmid DNA washed using WN buffer was higher than that of water with 1.87. The standard deviation was relatively small with 0.10. The average A260/A30 ratio of plasmid DNA washed using 75% ethanol was similar to that of WN buffer with 1.90 and standard deviation was 0.25. | |||
[[Image:A260-A230_ratio.png]] | |||
''''NanoDrop Results - Absorbance Spectra of plasmid DNA''' | |||
The values of concentration, A260/A280, A260/A230 of plasmid DNA samples used to draw the graphs shown above were all derivations from the absorbance spectrum of the plasmid DNA samples. However, only appreciating the values might be misleading in such ways as the same absorbance ratio could have been produced by the components of a sample that shows complete absence of plasmid DNA. Therefore, in order to confirm that the concentration and absorbance ratios have been established by plasmid DNA and other components that we expect to exist in our samples, such as proteins and possible contaminants, we have to take a look at the shape of the absorbance spectra. If the shape of our absorbance spectra shows great similarities with the typical absorbance spectrum of pure DNA sample, we can confirm the existence of plasmid DNA in our samples. | |||
Comparing these graphs with the typical absorbance spectrum of pure DNA (Figure 4. A) we can observe that the absorbance spectrum of DNA washed using WN buffer (Figure 4. B) and 75% ethanol (Figure 4. C) is similar to Figure 4. A. However absorbance spectrum of DNA washed using water (Figure 4. D) deviates relatively significantly from the typical absorbance spectrum of pure DNA (Figure 4. A) - the peak and trough absorbance of the spectra is distorted and unclear. From this we can know that it is highly likely that the absorbance spectra are produced indeed by the DNA present in our samples. To further endorse our assumption that plasmid DNA is the main source of absorbance, we can visualize the actual plasmid DNAs with gel electrophoresis (results are not shown), since the bands are the result of DNA shifting across the gel. | |||
[[Image:Absorbance_Spectra.png]] | |||
==DISCUSSION== | |||
We have set ddH2O as the negative control of this investigation, and we have expected that ddH2O will fail to give results that suggests high purification of plasmid DNA, in contrast to WN buffer. The results we acquired do in fact support our assumption, because the yield, or the average concentration of plasmid eluent washed with ddH2O was 6.5ng/ul, which is around 5 times lower than with WN buffer, and around 7 times lower than with ethanol (Figure 1.) The results are explainable through appreciating that the elution buffer (EB) can be replaced by water, indicating water also serves to elute out the plasmid and other contaminants on the membrane. Since we have used ddH2O to wash the supernatant, the discarded flow-through is likely to contain high concentration of plasmid DNA, RNA, proteins and other contaminants. Therefore, the amount of plasmid DNAs which still remain bound to the membrane and eluted out with EB is considerably less than the other two plasmid eluents. | |||
The results suggest that 75% ethanol was more effective than WN buffer in plasmid DNA extraction in terms of yield as the concentration of DNA in the extract washed with 75% ethanol was 46.65ng/ul, which was higher than the 34.5ng/ul obtained from the extract washed with WN buffer (Figure. 1.) This deviates from the theory that WN buffer must theoretically produce greater yield than ethanol. One possible explanation for the deviation is within the procedure to transfer the supernatant into the column. This process required each member to pipette up as much supernatant as possible without taking in the white precipitate. This meticulous method is prone to many variation to the investigation due to the individual discrepancy for determining the maximum amount, position and amount of white pellet, and the possibility for the white pellet to also be sucked into the tip. Ultimately, the plasmid DNA in the plasmid eluent washed with WN buffer and ethanol was higher in ethanol than in WN buffer because of human error derived from an inappropriate method. This explanation is supported by the high standard deviation for WN buffer and ethanol, although the mean values of yield for WN buffer and ethanol differs by more than 1 sd. Knowing the maximum volume of extraction of supernatant was 0.8ml, we could have just extracted 0.5ml, which is a much suitable method for analytical purposes, because it reduces the risk of absorbing the white pellet and most importantly fixes the total amount of supernatant to put into the column, thus allowing constant amounts of plasmid DNA. | |||
Our results have shown that 75% ethanol and WN buffer are similar in terms of the purity of plasmid DNA extract as samples washed with 75% ethanol obtained an average A260/A280 value of 1.89, whereas samples washed with WN buffer obtained an average A260/A280 value of 1.93(Figure 2). One possible reason for a slightly higher A260/A280 ratio for samples washed with WN buffer could be due to the chaotropic salts present in WN buffer. Chaotropic salts are known to increase the polarity of the solutions and create a salt bridge between the negatively charged nucleic acids and the less negatively charged silica membrane. As such, the samples washed with WN buffer would have a higher concentration of DNA and RNA adsorbed on the membrane. This would mean that the effect of a possible protein contamination on lowering the A260/A280 ratio is outweighed by the increased concentration of DNA and RNA in the final extract. Since the samples washed with ethanol did not have chaotropic salts, the A260/A280 ratio of samples washed with ethanol would be more towards the protein A260/A280 ratio which is lower than 1.8 than the samples washed with WN buffer. Another reason for the slight difference is because the presence of chaotropic salt, which is absent in ethanol, but present in the WN buffer can help the removal of protein by denaturing the protein. denatured protein in the WN sample would be able to flow through the membrane during the washing step while the non-denatured protein in the ethanol sample will be stuck on the membrane and therefore eluted out with the plasmid DNA.Protein has high absorbance at A280 and therefore the ratio for ethanol sample could have been smaller than the WN sample. The difference is, however, not very significant probably because significant amount of protein, along with other cell debris, was already removed by centrifuging step after adding MX3. | |||
In addition, since the A260/A280 ratios of 1.89 and 1.93 are higher than the A260/A280 ratio of a pure DNA sample, RNA contamination of the final extracts can be suspected. One possible reason for this could be that the MX1 buffer in the kit was kept in the fridge for more than a year, and hence the RNAase in MX1 buffer may not have been fully functional, thus being unable to completely degrade RNA in the samples. Therefore, undegraded RNA remains on the membrane of the columns used, and the samples taken for nanodrop were contaminated, causing the A260/A280 ratios to be higher than the expected ratio of 1.8. The similarity of 75% ethanol and WN buffer in terms of purity are also corroborated by the results of A260/A230 ratios, where samples washed with WN buffer obtained A260/A230 ratio of 1.87 and samples washed with WN buffer obtained A260/A230 ratio of 1.90. Again, the slightly higher ratio for WN buffer could be a consequence of the presence of chaotropic salts in WN buffer. | |||
A low A260/A230 ratio (below 1.8) indicates presence of contaminants that absorb 230nm. These are usually phenol residual or other organic contaminants. Since the ratio of plasmid DNA extract washed with water during washing step of the mini-preparation is significantly lower than that of WN buffer and 75% ethanol sample, it shows that WN buffer and ethanol are necessary components in washing step during plasmid extraction. The low ratio for plasmid DNA washed with water compared to those of WN buffer and 75% ethanol can also be explained by the difference in amount of nucleic acid. From figure 1. the yield of DNA sample is significantly lower for water than for WN or ethanol sample. This could have led to less 260 nm absorbance by nucleic acid and lowered the A260/A230 ratio for water. The small differences in average value of A260/A230 ratio and the completely overlapping error bars between 75% ethanol and WN buffer sample show that ethanol and WN buffer are not significantly different in their efficiency of removing organic contaminants during plasmid extraction. | |||
==METHOD== | |||
'''Plasmid Extraction''' | |||
Pcmv/myc/mito/GFP plasmid DNA was extracted from bacteria using conventional plasmid extraction protocol provided by VIogene except for the one of the washing steps. During the washing step, either WN buffer, water, ethanol was applied. Yield and purity of DNA sample were measured using NanoDrop, which provided DNA concentration and absorbance at A280, A260, and A230. | |||
'''DNA Gel Electrophoresis''' | |||
Gel electrophoresis was performed to verify the presence of plasmid DNA. 0.8% agarose gel was used for running the gel. Extracted Pcmv/myc/mito/GFP plasmid DNA was digested using Pst1 and Not1 restriction enzymes and loaded on the gel. | |||
==CONCLUSION== | |||
In conclusion,the comparison of DNA concentrations, A260/A280 ratios and A260/A230 ratios helped justify the evaluation of the quality and yield of plasmid DNA extraction using 75% ethanol and WN buffer from the Viogene kit. Based on the results obtained, it can be concluded that 75% ethanol was more effective than WN buffer in terms of yield of DNA, and that 75% ethanol and WN buffer resulted in similar purity of DNA extract from the samples. It is important to note, however, that the results obtained were influenced by several limitations of the experiment, including the use of old MX1 buffer, discrepancies among replicas during the transfer of supernatant after the addition of MX3 buffer, as well as a limited number of replicas for the experiment. Eliminating these limitations could have enhanced the accuracy of the results. | |||
==REFERENCE== | |||
K.D. Collins. (Jan, 1997). Charge density-dependent strength of hydration and biological structure. Biophysical Journal. Retrieved June 8, 2014, from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1184297/ | |||
Viogene-Biotek Corporation. (n.d.). Viogene-Biotek Corporation. Retrieved June 8, 2014, from http://www.viogene.com/ | |||
Viogene-Biotek Corporation. (n.d.). Viogene-Biotek Corporation. Retrieved June 8, 2014, from http://www.viogene.com/faq.php?fc_sn=1 | |||
Revision as of 20:55, 7 June 2014
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page | |
Entry titleINTRODUCTIONOur investigation involved mini-preparation, which is a rapid, small-scale extraction of plasmid from bacteria. Viogene kit, which is used for this process contains a solution called WN buffer. The main purpose of WN buffer is to remove protein and degraded RNA residues from the membrane. It consists of 75% to 80% ethanol and chaotropic salts, which enhances the removal of degraded RNA residue. The main objective of our investigation was to use this viogene kit to do plasmid extraction and in the process observe whether WN buffer or 75% ethanol gives higher DNA yield and purity. Yield was determined by the concentration of DNA (ng/ml) and purity of DNA was determined by measuring A260/A280 and A260/230 ratio and observing how far this ratio deviates from the optimal purity ratio. DNA absorbs at both 260nm and 280nm whereas protein only absorbs at 280nm. So, a pure sample of DNA will have A260/A280 ratio around 1.8 and below 1.8 identifies possible protein contamination. Similarly organic contaminants such as phenol absorb light of 230nm wavelength whereas nucleic acids absorb at 260nm. So A260/A230 ratio can also tell us about the purity level of plasmid DNA.
OBJECTIVESCompare the quality and the yield of the plasmid DNA extracted and washed using 75% ethanol and WN buffer from the Viogene kit.
EXPERIMENT DESIGNWe used three different samples to clean the extracted plasmid DNA: water, 75% ethanol and WN buffer provided by the Viogene kit. Water served as a negative control and WN buffer served as a positive control. 75% ethanol was tested to see how much yield and purity of DNA it gives and we compared this with the yield and purity of DNA WN buffer gives. With each person of our group carrying out the full experiment, we obtained 4 sets of data for yield and purity of DNA extract
RESULTSNanoDrop Results - Yield, A280/A260 an A260/A230 ratio of plasmid DNA The average concentration of plasmid DNA sample washed using water during the wash step was lowest with 6.5ng/ml and with standard deviation of 2.8. The average concentration of plasmid DNA washed using WN buffer was around five times that of water with 34.5ng/ml. The standard deviation, however, was also around fives times bigger with 10.4. The average concentration of plasmid DNA washed using 75% ethanol was highest with 46.65ng/ml and standard deviation of 12.9. The error bars for ethanol and WN buffer sample overlap.
'NanoDrop Results - Absorbance Spectra of plasmid DNA The values of concentration, A260/A280, A260/A230 of plasmid DNA samples used to draw the graphs shown above were all derivations from the absorbance spectrum of the plasmid DNA samples. However, only appreciating the values might be misleading in such ways as the same absorbance ratio could have been produced by the components of a sample that shows complete absence of plasmid DNA. Therefore, in order to confirm that the concentration and absorbance ratios have been established by plasmid DNA and other components that we expect to exist in our samples, such as proteins and possible contaminants, we have to take a look at the shape of the absorbance spectra. If the shape of our absorbance spectra shows great similarities with the typical absorbance spectrum of pure DNA sample, we can confirm the existence of plasmid DNA in our samples. Comparing these graphs with the typical absorbance spectrum of pure DNA (Figure 4. A) we can observe that the absorbance spectrum of DNA washed using WN buffer (Figure 4. B) and 75% ethanol (Figure 4. C) is similar to Figure 4. A. However absorbance spectrum of DNA washed using water (Figure 4. D) deviates relatively significantly from the typical absorbance spectrum of pure DNA (Figure 4. A) - the peak and trough absorbance of the spectra is distorted and unclear. From this we can know that it is highly likely that the absorbance spectra are produced indeed by the DNA present in our samples. To further endorse our assumption that plasmid DNA is the main source of absorbance, we can visualize the actual plasmid DNAs with gel electrophoresis (results are not shown), since the bands are the result of DNA shifting across the gel.
DISCUSSIONWe have set ddH2O as the negative control of this investigation, and we have expected that ddH2O will fail to give results that suggests high purification of plasmid DNA, in contrast to WN buffer. The results we acquired do in fact support our assumption, because the yield, or the average concentration of plasmid eluent washed with ddH2O was 6.5ng/ul, which is around 5 times lower than with WN buffer, and around 7 times lower than with ethanol (Figure 1.) The results are explainable through appreciating that the elution buffer (EB) can be replaced by water, indicating water also serves to elute out the plasmid and other contaminants on the membrane. Since we have used ddH2O to wash the supernatant, the discarded flow-through is likely to contain high concentration of plasmid DNA, RNA, proteins and other contaminants. Therefore, the amount of plasmid DNAs which still remain bound to the membrane and eluted out with EB is considerably less than the other two plasmid eluents. The results suggest that 75% ethanol was more effective than WN buffer in plasmid DNA extraction in terms of yield as the concentration of DNA in the extract washed with 75% ethanol was 46.65ng/ul, which was higher than the 34.5ng/ul obtained from the extract washed with WN buffer (Figure. 1.) This deviates from the theory that WN buffer must theoretically produce greater yield than ethanol. One possible explanation for the deviation is within the procedure to transfer the supernatant into the column. This process required each member to pipette up as much supernatant as possible without taking in the white precipitate. This meticulous method is prone to many variation to the investigation due to the individual discrepancy for determining the maximum amount, position and amount of white pellet, and the possibility for the white pellet to also be sucked into the tip. Ultimately, the plasmid DNA in the plasmid eluent washed with WN buffer and ethanol was higher in ethanol than in WN buffer because of human error derived from an inappropriate method. This explanation is supported by the high standard deviation for WN buffer and ethanol, although the mean values of yield for WN buffer and ethanol differs by more than 1 sd. Knowing the maximum volume of extraction of supernatant was 0.8ml, we could have just extracted 0.5ml, which is a much suitable method for analytical purposes, because it reduces the risk of absorbing the white pellet and most importantly fixes the total amount of supernatant to put into the column, thus allowing constant amounts of plasmid DNA. Our results have shown that 75% ethanol and WN buffer are similar in terms of the purity of plasmid DNA extract as samples washed with 75% ethanol obtained an average A260/A280 value of 1.89, whereas samples washed with WN buffer obtained an average A260/A280 value of 1.93(Figure 2). One possible reason for a slightly higher A260/A280 ratio for samples washed with WN buffer could be due to the chaotropic salts present in WN buffer. Chaotropic salts are known to increase the polarity of the solutions and create a salt bridge between the negatively charged nucleic acids and the less negatively charged silica membrane. As such, the samples washed with WN buffer would have a higher concentration of DNA and RNA adsorbed on the membrane. This would mean that the effect of a possible protein contamination on lowering the A260/A280 ratio is outweighed by the increased concentration of DNA and RNA in the final extract. Since the samples washed with ethanol did not have chaotropic salts, the A260/A280 ratio of samples washed with ethanol would be more towards the protein A260/A280 ratio which is lower than 1.8 than the samples washed with WN buffer. Another reason for the slight difference is because the presence of chaotropic salt, which is absent in ethanol, but present in the WN buffer can help the removal of protein by denaturing the protein. denatured protein in the WN sample would be able to flow through the membrane during the washing step while the non-denatured protein in the ethanol sample will be stuck on the membrane and therefore eluted out with the plasmid DNA.Protein has high absorbance at A280 and therefore the ratio for ethanol sample could have been smaller than the WN sample. The difference is, however, not very significant probably because significant amount of protein, along with other cell debris, was already removed by centrifuging step after adding MX3. In addition, since the A260/A280 ratios of 1.89 and 1.93 are higher than the A260/A280 ratio of a pure DNA sample, RNA contamination of the final extracts can be suspected. One possible reason for this could be that the MX1 buffer in the kit was kept in the fridge for more than a year, and hence the RNAase in MX1 buffer may not have been fully functional, thus being unable to completely degrade RNA in the samples. Therefore, undegraded RNA remains on the membrane of the columns used, and the samples taken for nanodrop were contaminated, causing the A260/A280 ratios to be higher than the expected ratio of 1.8. The similarity of 75% ethanol and WN buffer in terms of purity are also corroborated by the results of A260/A230 ratios, where samples washed with WN buffer obtained A260/A230 ratio of 1.87 and samples washed with WN buffer obtained A260/A230 ratio of 1.90. Again, the slightly higher ratio for WN buffer could be a consequence of the presence of chaotropic salts in WN buffer. A low A260/A230 ratio (below 1.8) indicates presence of contaminants that absorb 230nm. These are usually phenol residual or other organic contaminants. Since the ratio of plasmid DNA extract washed with water during washing step of the mini-preparation is significantly lower than that of WN buffer and 75% ethanol sample, it shows that WN buffer and ethanol are necessary components in washing step during plasmid extraction. The low ratio for plasmid DNA washed with water compared to those of WN buffer and 75% ethanol can also be explained by the difference in amount of nucleic acid. From figure 1. the yield of DNA sample is significantly lower for water than for WN or ethanol sample. This could have led to less 260 nm absorbance by nucleic acid and lowered the A260/A230 ratio for water. The small differences in average value of A260/A230 ratio and the completely overlapping error bars between 75% ethanol and WN buffer sample show that ethanol and WN buffer are not significantly different in their efficiency of removing organic contaminants during plasmid extraction.
METHODPlasmid Extraction Pcmv/myc/mito/GFP plasmid DNA was extracted from bacteria using conventional plasmid extraction protocol provided by VIogene except for the one of the washing steps. During the washing step, either WN buffer, water, ethanol was applied. Yield and purity of DNA sample were measured using NanoDrop, which provided DNA concentration and absorbance at A280, A260, and A230.
CONCLUSIONIn conclusion,the comparison of DNA concentrations, A260/A280 ratios and A260/A230 ratios helped justify the evaluation of the quality and yield of plasmid DNA extraction using 75% ethanol and WN buffer from the Viogene kit. Based on the results obtained, it can be concluded that 75% ethanol was more effective than WN buffer in terms of yield of DNA, and that 75% ethanol and WN buffer resulted in similar purity of DNA extract from the samples. It is important to note, however, that the results obtained were influenced by several limitations of the experiment, including the use of old MX1 buffer, discrepancies among replicas during the transfer of supernatant after the addition of MX3 buffer, as well as a limited number of replicas for the experiment. Eliminating these limitations could have enhanced the accuracy of the results.
REFERENCEK.D. Collins. (Jan, 1997). Charge density-dependent strength of hydration and biological structure. Biophysical Journal. Retrieved June 8, 2014, from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1184297/ Viogene-Biotek Corporation. (n.d.). Viogene-Biotek Corporation. Retrieved June 8, 2014, from http://www.viogene.com/ Viogene-Biotek Corporation. (n.d.). Viogene-Biotek Corporation. Retrieved June 8, 2014, from http://www.viogene.com/faq.php?fc_sn=1
| |