User:John M.L. Craven/Notebook/20.109 Mod 3, W/F Green Presentation
Optimization of Extracellular Electron Transfer in Microbial Fuel Cells Using Geobacter sulfurreducens
Big Picture
With increasing concerns in society about the depletion of fossil fuels and the harmful effects of carbon dioxide in the atmosphere, there is a great need for sources of energy from biological, renewable matter that will produce electricity and not be harmful to the environment. Microbial fuel cells, which use microorganisms to convert organic matter into fuel, may be a solution to this need if the process can be optimized for most applications of the technology. The objective of our project is to optimize the extracellular electron transfer capabilities of Geobacter sulfurreducens in microbial fuel cells. This will result in a more efficient process that can produce energy on a scale more competitive with current technologies.
Specific Aims
- Create a genome scale metabolic network model of G. sulfurreducens in silico -- Constraint Based Modeling
- Study the intracellular dynamics of the G. sulfurreducens -- Metabolic Flux Analysis
- Determine the gene deletions that optimize extracellular electron transfer -- Optimization
- Examine genome wide effects of these deletions in vitro -- DNA Microarray Analysis
Background -- Microbial Fuel Cells (MFCs)
In recent years, there has been increasing concern about the supplies of fossil fuels dwindling and the harmful effects of carbon dioxide in the atmosphere. There is a need for sources of energy from renewable matter that will not harmfully affect the environment. Microbial fuel cells that produce electricity from organic matter may be an answer to this problem. Microbial fuel cells (MFCs) allow for the sustainable production of energy from biodegradable, reduced compounds. The production of electricity from organic matter using microbial fuel cells is hardly a new concept- the principle dates back nearly a century. Upon development of the idea over the past 100 years, researchers have been interested in improving the power output of the MFCs in order to increase applications on a larger scale. However, there is very limited information about the energy metabolism and the nature of the bacteria involved in the process. In addition, the precise electron transfer mechanisms have yet to be established. This knowledge is essential in order to appropriately optimize energy production.
An MFC converts the energy available in a bio-convertible substrate directly into electricity. MFCs function by oxidizing an electron donor with electron transfer to the anode under anoxic conditions. The electron donor can be a reduced product of microbial metabolism or an added artificial mediator that serves to facilitate electron transfer between the microbe and the anode. Some microorganisms can produce their own soluble electron transfer mediator, and some microorganisms are capable of directly transferring electrons to the anode surface. The electricity generation is achieved when bacteria switch from using a natural electron acceptor such as oxygen or nitrate to an insoluble electron acceptor such as the MFC anode. Overall, the substrate is metabolized by the bacteria, which transfer the gained electrons to the anode. The electrons are diverted to the anode via the use of soluble electron shuttles or through membrane-bound electron shuttling compounds. The electrons then flow from the anode, through a resistor, and to the cathode, as shown in Figure 1. The cathode is either exposed to air or submerged in aerobic water. This ultimately creates electrical current and an off-gas of primarily carbon dioxide. Electrons, protons, and oxygen combine at the cathode surface to form water. The metabolic pathways that determine the flow of electrons and protons are essential to the generation of the electricity.
There are several advantages to MFCs over other current technologies that use organic matter to generate energy. The direct conversion of substrate energy into electricity allows for high conversion efficiency. MFCs do not require energy input or gas treatment of any kind. MFCs are able to operate effectively at ambient or low temperatures, unlike many bio-energy processes. They also have widespread application in places that do not have electrical infrastructure. Lastly, MFCs add to the diversity of fuel types that we can use to meet global energy needs.
Several important biological and electrochemical parameters define the performance of MFCs. First, the substrate conversion rate depends largely on bacterial kinetics and the potential over the MFC. Second, the overpotentials at the anode and the cathode are determined by the electrode surface, the electrochemical characteristics of the electrode, and the electrode potential. Third, the performance of the proton exchange membrane can alter the MFC internal resistance. Lastly, the internal resistance of the MFC is dependent on both the membrane resistance and the resistance of the electrolyte between the electrodes. The optimal conditions are met when the anode and cathode are as close together as possible.
Thus far, there have been biological and technological improvements made to optimize performance of the MFCs. Biological optimization include selection of suitable bacteria and bacterial adaptation to optimal reactor conditions. Technological optimization is done primarily through the addition of soluble redox mediators to the anode and to an increase in anode surface. Furthermore, improved anode materials can be developed by using chemical catalysts. Recently, researchers are better able to design MFCs that can compete with conventional power sources. Scientists are also able to engineer better systems capable of harvesting electricity from organic wastes. In addition, many microorganisms have been discovered that are strongly capable of sustained, efficient electricity production.
Background -- Geobacter sulfurreducens
A recent development in the study of MFCs was the development of a cell that can harvest electricity from organic matter in aquatic sediments. The apparatus consists of an anode buried in anoxic marine sediments and connected to a cathode suspended in the aerobic water above. These designs can be set up in remote locations to harvest electricity from places such as compost piles, septic tanks, and waste lagoons. It was believed that microbes degrade organic matter in sediments and produce reduced end products such as sulfide and Fe2+. Then the reduced end products were the direct source of electrons and donated the electrons to the electrodes. These aquatic sediment studies actually showed that the microorganisms themselves were more directly involved in the electron transfer to the anode.
Analysis of the microbes on the anode surfaces showed that organisms of the family Geobacteraceae comprised half of all the organisms present. Geobacter species were most abundant on electrodes in freshwater sediments. Studies showed that Geobacteraceae can conserve energy to support growth by completely oxidizing acetate and other organic compounds to carbon dioxide with an electrode serving as the electron acceptor. This ability to produce electricity related to their ability to transfer electrons to Fe3+ and Mn4+ oxides- insoluble, extracellular electron acceptors. The microorganisms catalyze the oxidation of sediment organic matter coupled to the reduction of Fe3+ and Mn4+ oxides. The organic matter is broken down to produce fermentation products such as acetate, aromatic compounds, and long-chain fatty acids- electron donors for Geobacteraceae. The Geobacteracease oxidize these electron donors and reduce Fe3+ or Mn4+.
Electron transfer in Geobacteraceae has primarily been evaluated using Geobacter sulfurreducens. This organism is has been used for several main reasons: 1) closely related microorganisms have been found to be the predominant organisms on the anodes of fuel cells harvesting electricity from aquatic sediments and complex wastes; 2) the complete genome sequence, genetic system, genome microarrays, and substantial physiological data are available; 3) the metabolism of G. sulfurreducens can be tracked by monitoring the gene transcript levels and expression while growing on electrodes; 4) metabolism can be altered to increase respiration rates and lead to increased electricity production; 5) Geobacter species have the unique ability to completely oxidize organic substrates to carbon dioxide with an anode serving as the sole electron acceptor; and 6) in systems that harvest electricity in highly anoxic conditions, the Geobacter species accounts for over 70% of the microbes on the anode surface. In addition, the Geobacter species can accept electrons from an electrode if the electrode is placed at a negative potential. Pure cultures of Geobacter can reduce physiological electron acceptors such as nitrate and fumarate with the electrode serving as the electron donor. Overall, G. sulfurreducens allow researchers to gain insight about the mechanisms of electron transfer to anodes and how best to optimize the process through fuel cell design or genetic engineering.
Background -- Extracellular Electron Transfer
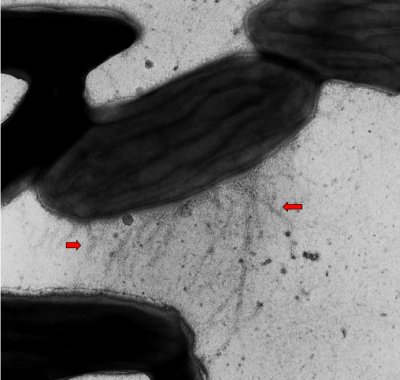
There is a need for significant optimization of microbial fuel cells for most applications of the technology. In order for the optimization to be carried out, the transfer of electrons to electrodes needs to be evaluated, as well as the physiology and ecology of the microbes. Extracellular electron transfer, the property of the microbial fuel cell that we seek to optimize, is defined as the process in which electrons are 1) derived from the oxidation of electron donors, 2) transferred to the outer surface of the cell, and 3) reduce an extracellular terminal electron acceptor. According to Lovely, extracellular electron transfer to minerals such as Fe(III) and Mn(IV) oxides and to electrodes is currently the greatest interest in the research arena because of the technological applications and current significance to our environment. The present methods for harvesting electricity from organic wastes couple the oxidation of organic matter to the electron transfer to electrodes. Currently, researchers are evaluating how the electrons are transferred to the electron acceptors and what factors control the rate at which this occurs. To name a few areas of uncertainty that scientists are trying to elucidate: the role of “microbial nanowires” (see below images) emanating from microorganisms as the conduit for electron transfer; whether microorganisms such as Geobacter have “iron lungs” that serve as capacitors to store electrons when electron acceptors are not available; the mechanism by which electrons pass through the biofilms on the anodes of microbial fuel cells; and, whether metal-reducing microorganisms adapt a particular physiological state in a microbial fuel cell. These are just a few of the many topics about extracellular electron transfer that can shed insight on the best ways to optimize the process.
Papers
- Microbial Fuel Cells
Rabaey, K. & Verstraete, W. (2005). Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol 23(6): 291-8.
Shukla AK, Suresh P, Berchmans S, Rahjendran A: A Biological Fuel cell and their applications. Curr Sci 2004, 87:455-468.
Lovley, D. R. (2006). Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol 17, 327-32.
Roller, S.D. et al. (1984) Electron-transfer coupling in microbial fuel-cells. Comparison of redox-mediator reduction rates and respiratory rates of bacteria. J. Chem. Technol. Biotechnol. B Biotechnol. 34, 3-12.
Larminie, J. and Dicks, A. (2000) Fuel cell systems explained, John Wiley & Sons.
Park, D.H. and Zeikus, J.G. (2003) Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol. Bioeng. 81, 348-355.
Reimers CE, Tender LM, Fertig S, Wang W: Harvesting energy from the marine sediment-water interface. Environ Sci Technol 2001, 35:192-195.
Gregory GB, Bond DR, Lovely DR: Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 2004, 6:596-604.
- Geobacter sulfurreducens
Bond DR, Lovely DR: Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 2003, 69:1548-1555.
Nevin, KP et al. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environmental microbiology (2008).
B. A. Methe et al., “Genome of Geobacter sulfurreducens: metal reduction in subsurface environments”, Science 302 (5652) 1967 (2003).
A. S. Galushko and B. Schink, “Oxidation of acetate through reactions of the citric acid cycle by Geobacter sulfurreducens in pure culture and in syntrophic coculture”, Arch. Microbiol. 174 (5), 314 (2000).
- Extracellular Electron Transfer
Lovley, D.R. 2008. Extracellular electron transfer: wires, capacitors, iron lungs, and more. Geobiology 6(3):225-231.
- Modeling
S. Becker et al., “Quantitative prediction of cellular metabolism with constraint-base models: The COBRA Toolbox”, Nat. Protoc. 2, 727 (2007).
R. Mahadevan et al., “Characterization of Metabolism in the Fe (III)-Reducing Organism Geobacter sulfurreducens by Constraint-Based Modeling”, Applied and Environmental Microbiology 72 (2), 1558 (2006).
- Microarray
DE Holmes, SK Chaudhuri, KP Nevin, T Mehta, BA Methé, A Liu, JE Ward, TL Woodard, J Webster, DR Lovley. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environmental Microbiology, 8(10):1805-1815 (2006)