User:Kari Anna Byrnes/Notebook/Biology 210 at AU
February 1, 2015 - KB Lab #3 Microbiology and Identifying Bacteria with DNA Sequences
The purpose of this lab was to learn more about the identification and characteristics of bacteria. It also displayed the effects of antibiotic resistance. PCR was used to understand how DNA sequences are used to identify species.
Last week, we prepared serial dilutions. To do that, we obtained four nutrient agar plates and four tetracycline (antibiotic) plates; each plate was labelled. Each of the four plates were coated with different dilutions of the water in the hay infusions + sterile broths. Using micropipettors set at 100 microliters, we added the culture + mixed (each different dilution 10^-2, 10^-4, 10^-6, 10^-8) of broth to the plates. The agar plates then incubated in room temperature for a week. We observed our hay infusion culture: it's water level was much lower, it smelled less potent than a week ago, and the top film had disappeared. I attributed the water loss and nutrient loss to have killed many of the species in the jar. After that week, we gathered our agar plates and observed that bacteria had grown on each plate. We counted the amount of colonies (or a lawn) on each of the plates and recorded it on a table. Next, we made two wet mounts of the bacteria grown on the nutrient agar and tetracycline plates. We scraped up a bit of the colony, added it onto a slide along with a drop of water and coverslip on top. We then observed the slide under the microscope and recorded our findings. Our next procedure was the gram stains. On four labelled slides, we placed samples of TET bacteria 10^-5, TET bacteria 10^-7, Nutrient bacteria 10^-3, and Nutrient bacteria 10^-7. After adding a drop of water to each slide, we heated it by passing it under a flame 3 times. From there, we added crystal violet for 1 minute. Rinsed with water. Gram's iodine for 1 minute. Rinsed with water. 95% alcohol for 15 seconds. Safranin stain for 30 seconds. Rinsed with water. We blotted the extra water off then let the samples fully air dry. Then we looked at each slide under microscopes and recorded what we found. We then prepared a PCR for 16S sequencing. A single colony of bacteria was transferred to a 100 microliter water in a sterile tube and incubated at 100 celsius for 10 minutes, Then the sample was centrifuged for 5 minutes as 13,400 rpm. The supernatant was then added to a PCR tube mixed with 20 microliters of primer/water and added into the PCR machine. Next week we'll put it in an agarose gel and test for sequencing.
This is what the bacteria sample looked like from the Tetracycline agar under the microscope.
This is what the bacteria sample looked like from the Nutrient agar under the microscope.



 Figure 4
This is our TET bacteria 10^-5 under the microscope at 10x.
It is non motile, gram positive, purple and blue. It contains dots and large circular bacteria.
Figure 4
This is our TET bacteria 10^-5 under the microscope at 10x.
It is non motile, gram positive, purple and blue. It contains dots and large circular bacteria.
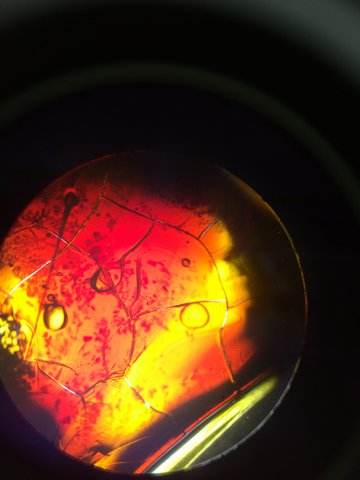 Figure 5
This is our TET bacteria 10^-7 under the microscope at 10x.
It is non motile, gram negative, resembles orange and yellow colored shards of glass, and contains circular bacteria.
Figure 5
This is our TET bacteria 10^-7 under the microscope at 10x.
It is non motile, gram negative, resembles orange and yellow colored shards of glass, and contains circular bacteria.


Our findings have led us to believe that our bacteria are greatly influenced by antibiotics, yet others are not. By looking at the gram stain, we can tell which of our bacteria contains the most peptidoglycan, which can further identify our bacteria. When we see the PCR results, we will be able to accurately identify our bacteria from our transect.
January 28, 2015 - KB Lab #2 Identifying Algae and Protists
The purpose of this lab was to learn to differentiate the different types of protists and algae. We learned to identify the protists with the dichotomous key. The key classified unicellular organisms based on their size shape, movement, and color. I hypothesize that each of our hay infusion cultures from each transect will contain different organisms. To test this, we will take samples from our culture and identify the types of protists under the microscope.
I grabbed our hay infusion culture that was prepared last class. The culture contained microorganisms in each part of it. There were different organisms with different niches in each part of the culture. Since our jar consisted of different parts, we pipetted samples from the top and bottom. From the samples, we created wet mounts used for the microscope. We then identified and recorded three organisms from each part of the culture (top and bottom) using the dichotomous key.
When we observed our hay infusion culture which sat out for a week, it was clear that there were different ecosystems within the jar. The culture smelled moldy, stale, and pond-like. There was a dark green moldy, floating substance on the top. The middle section was greenish, yet transparent. There was a green mold that had settled on the bottom of the jar. Although we could not see anything moving with our naked eyes, we assumed there were living protists in the water and moldy substances.
The wet mount of the top was examined. Using the dichotomous key, we identified paramecium bursarla, which was motile and about 120 micrometers large. Next we found didinium, a stationary, unicellular protist around 80 micrometers. Lastly we found a motile green algae called pandorina that was 30 micrometers big. On the bottom sampled wet mount, we found paramecium aurelia, a motile, colorless 140 micrometer protist. Actinospharium, a green, 70 micrometer protist was also found. Finally, paramecium bursarla was also found on the bottom, yet it was smaller (80 micrometer) and still motile. All these organisms are living because they each require energy, are organized and complex, reproduce (asexually), and can grow.
The conclusions of this experiment clearly followed the purpose of the lab. We found a variety of protists and algae in our hay infusion cultures. The types of protists from the top and bottom wet mounts were different, suggesting that each ecosystem inside our jar required different types of protists. If this culture grew for another two months, we would most likely see most organisms due to meiosis and mitosis or we could see less organisms due to less resources the species need to survive.
January 25, 2015 - KB
Lab #1 Biological life at AU
The purpose of this lab was to observe an ecosystem. I was also required to collect a sample from the environment in that ecosystem. By observing a specific ecosystem, I am able to see the organisms and their niches in the community.
In this experiment, my partners and I were assigned a 20 m x 20 m transect (an ecosystem) called number 3: Tall bushes. We were required to draw a picture of our observations about the ecosystem. We found abiotic and biotic components of the section and labelled their location and identity. After perfecting our sketches and findings, we obtained a sterile 50 mL conical tube in which we placed a sample of soil/ ground vegetation from our transect. We returned to the classroom to perform a Hay Infusion Culture. We first took 10 g of our sample and placed it into a plastic jar filled with 500 mL of deerpark water. Next we added 0.1 g of dried milk, attached the lid of the plastic jar, and mixed the contents. Finally, the jar was labelled with our group name and placed on a table without the lid.
Our transect looked as followed:
My transect was labelled It is located in the center of campus, south of the amphitheater and east of the gymnasium. The section we are observing is flat. It is centered in an arboretum. It is an flat hourglass section, surrounded by sidewalks on all sides.
The components in transect 3 were: Abiotic Components: sidewalk, lamp post, snow Biotic Components: short shrubs, trees (deciduous), leaves, soil
This lab will lead me to find out more about my transect. The Hay Infusion Culture will display the types of organisms (protists) that make up transect 3. From there, we will be able to use a dichotomous key to find out the species and their niches in the community




