User:Klare Lazor/Notebook/Chem-496-001/2011/09/07
 Biomaterials Design Lab Biomaterials Design Lab
|
<html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> |
|
ObjectiveThe purpose of this experiment is to determine the ideal pH for the synthesis of Au nanoparticles. For this experiment, Au nanoparticles will be created by reducing HAuCl4 with bovine serum albumen (BSA) in two aqueous solutions. One with a pH of 7.55 and another with a pH of 7(water). Furthermore, the temperature range will be between 74-82 degrees Celsius. DescriptionProtocol: Materials needed included Chloroauric Acid (HAuCl4), bovine serum albumen (BSA), and zein protein. BSA(0.0015-0.015mM) and HAuCl4(0.25-10mM) were used to create 10mL aqueous mixtures. Each mixture was transferred to a screw-capped glass bottle. The mixtures were kept in a water thermostat bath a 40, 60, or 80±1˚C for 6 hours. The solutions changed from colorless to pink-purple and finally purple. The samples were then removed from the bath and left to cool overnight. Next, they were purified with distilled water two times in order to remove any excess BSA, using a centrifuge at 10,000-14,000rpm for five minutes. The samples were placed in different temperature baths to observe how temperature affects the synthesis of the gold nanoparticles. In addition, in order to determine the absorbance caused by surface plasmo resonance, UV-visible spectra were taken of each sample using an UV Spectrophotometer with a wavelength range of 200-900nm. Time dependent scans were also taken to collect data on the growth kinetics of AU nanoparticles. Other data was collected using a pH meter, SEM, TEM, X-ray diffraction and AFM. Last, zein protein film formation was carried out using the BSA conjugated nanoparticles. A clear solution of zein (10%w/v) in aqueous ethanol (90%v/v) along with glycerol (30% on zein weight basis) was prepared. Then, the BSA conjugated nanoparticles (10%v/v) was added into this solution. Five grams of this solution was placed in a petri dish and swirled to coat the dish. It was placed in an 40˚C oven for 24 hours to eventually lead to protein film formation. A texture analyzer was then used in order to determine the mechanical properties of the films containing different samples of BSA conjugated Au nanoparticles. (“Protein Films of Bovine Serum Albumen Conjugated Gold Nanoparticles: A Synthetic Route from Bioconjugated Nanoparticles to Biodegrable Protein Films” by Mandeep Singh Bakshi, Harpreet Kaur, Poonam Khullar, Tarlok Singh Banipa, Gurinder Kaur, and Narpinder Singh). Procedure: In a screw-capped glass bottle, 10mL of BSA(1.5mM) and HAuCl4(0.25mM) were added to 10mL of TRIS that was titrated to a pH of 7.55. The TRIS was already titrated by instructor. A UV-visible spectra was taken of the solution with a wavelength of 200-800nm, as well as of the buffer for a standard. The solution was then placed in a 74-82˚C water thermostat bath for 30 minute intervals. After each interval, a UV-visible spectra was taken of the solution to determine the absorbance caused by the surface plasmo resonance. This was repeated again, however the aqueous solution was water. Stock solutions were created by the instructor. DataCalculations: Were calculated by the professor. Similar calculations can be found in notebook from last week.
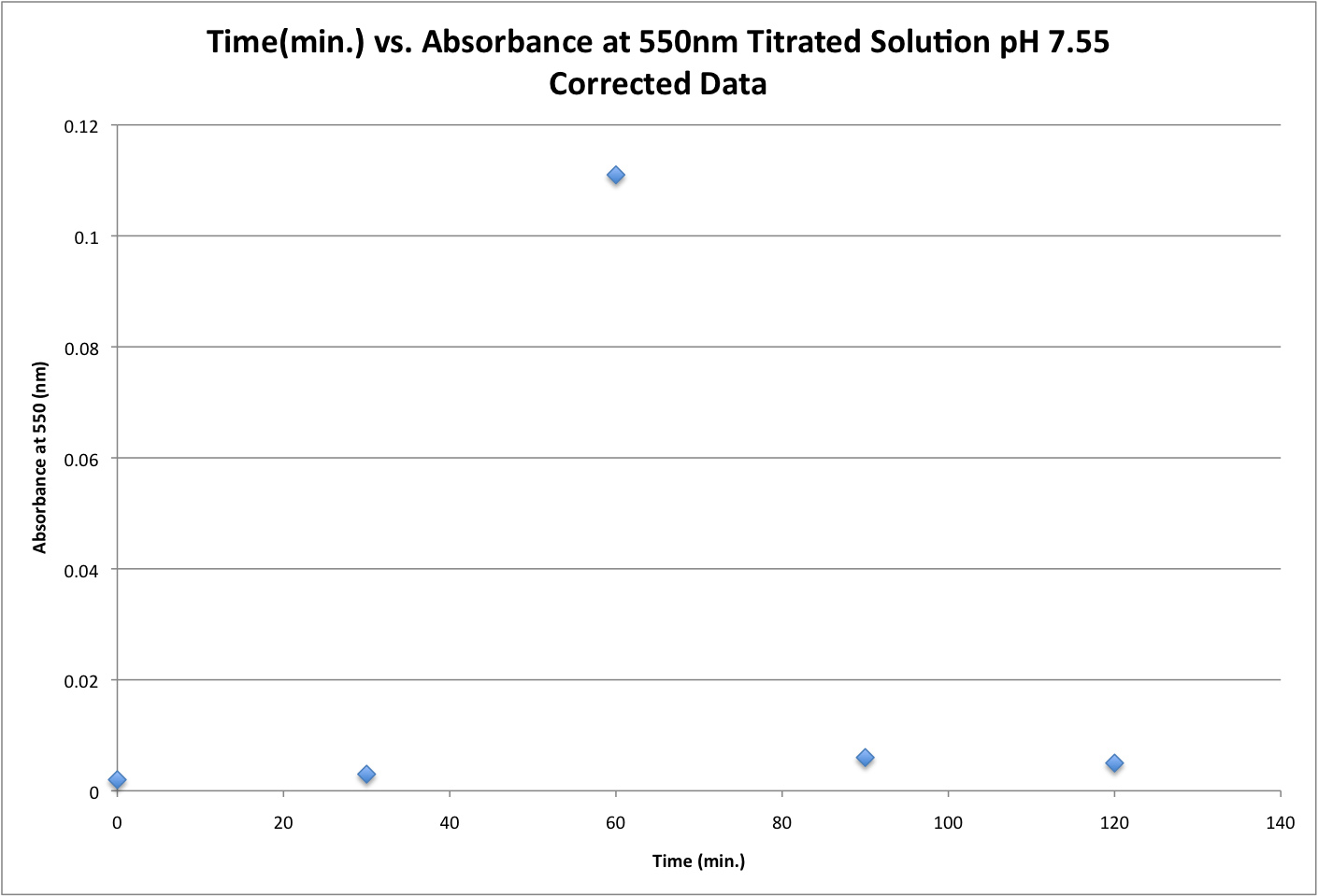
stock concentrations: chloric acid:2.5mM and bsa:15 micro molar new concentrations: Chloric acid: .25mM and bsa: 1.5 micro molar Image 1: Corrected data plotted at Time (minutes) vs. Absorbance at 550nm for aqueous solution with a pH of 7.55. Image 2: Corrected data plotted at Wavelength (nm) vs. Absorbance for aqueous solution with pH of 7.55. Series 1: T=0min, series 2: T=30min, series 3: T= 60 min, series 4: T= 90 min, and series 5: T=120 min.
Image 5: Corrected data plotted at Wavelength (nm) vs. Absorbance for aqueous solution with pH of 7 (water). Series 1: T=0min, series 2: T=30min, series 3: T= 60 min, series 4: T= 90 min, and series 5: T=120min.
NotesKlare, what do you make of your data? What do you think it means? Is this consistent with what Bashki et al see in their paper for nanoparticle synthesis? Matt Hartings 12:17, 14 September 2011 (EDT) Contents of Aqueous Solutions Actual Amounts
Results
Use categories like tags. Change the "Course" category to the one corresponding to your course. The "Miscellaneous" tag can be used for particular experiments, as instructed by your professor. Please be sure to change or delete this tag as required so that the categories remain well organized. | |