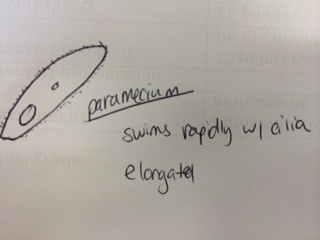User:Meredith Gleason1/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| Line 73: | Line 73: | ||
Returning to the consideration of the Gram slides made from these bacterial colonies (as described above), microscopy observations were made as to the shape and Gram positive or negative nature of the bacteria. Interestingly, the orange bacterial colonies present on the Tetracycline were found to be Gram-positive upon staining and the white colonies grown without the presence of Tetracycline were Gram-negative. Observations for the 3 most clear samples/slides were recorded in Table 2 (above). | Returning to the consideration of the Gram slides made from these bacterial colonies (as described above), microscopy observations were made as to the shape and Gram positive or negative nature of the bacteria. Interestingly, the orange bacterial colonies present on the Tetracycline were found to be Gram-positive upon staining and the white colonies grown without the presence of Tetracycline were Gram-negative. Observations for the 3 most clear samples/slides were recorded in Table 2 (above). | ||
Conclusions: The Hay Infusion Culture produced several varieties of bacteria, as | '''Conclusions:''' The Hay Infusion Culture produced several varieties of bacteria, as evidenced by the variety of colonies on the dilution series agar plates.Initial observations, both of the colonies and then of the stained bacteria under microscopy, revealed a presence of 2 possible distinct varieties - white and orange - that will be identified in a future project with 16s sequencing with PCR. Site 2 produced a variety of protists (see Lab 2) and bacteria to study in a laboratory setting. Both the presence of the protists and bacteria are expected in such an environment but nonetheless interesting and worth further identification, especially of the different bacteria and the antibiotic-resistant variety(ies) found in Site 2. | ||
Works cited: | Works cited: | ||
Revision as of 08:47, 10 July 2014
Lab 1: Biological Life at AU
Date: 7/1/14
Objective:This project focuses on a transect of land near the campus of American University in Washington, DC. While most would consider the campus to be an "urban environment", there is in fact quite a bit of green space available to analyze the ecosystems that exist on campus.
Methods/Observations: I traveled to the site of transect, Site #2, and made preliminary observations about the site. Site #2 is located on the grounds of the Wesley Theological Seminary, located just outside the northwestern boundaries of American University's campus. The transect of land, approximately 20x20 feet in size, is a wooded area with 6 pine trees with a dense area of ground cover below. Of the 6 trees, 3 are coniferous and 3 are deciduous. This transect is surrounded on all sides by grass but within the confines of the ~20x20 area of the trees, the ground is not covered with grass, but is instead covered with pine needles that have fallen and various short ground plants, like ivies and common weeds. An aerial view of the area is seen here:
And an eye-level photo is seen here:
At the site, an 11.6 g sample was taken, including soil found ~10 feet between two coniferous trees, along with pine needles and small leaves of ground ferns and ivies growing in the soil. Upon return to the lab, a Hay Infusion Culture was made with the entire soil sample, 0.1 g of dried milk, and 500 mL of filtered, Deerpark water in a large jar. The sample was vigorously mixed and left - with the lid open - on a lab bench to slowly separate.
Results & Conclusions: The result was, primarily, to make observations and set up a Hay Infusion Culture to be able to identify protists at a future date. Conclusions at this point are not appropriate as the infusion culture has not been studied.
______________________________________________
Lab 2: Identifying Algae and Protists
7/3/14
Objective: The objective of this laboratory investigation was to practice using the dichotomous key of possible protists to identify protists in a sample from the Hay Infusion Culture (produced in the previous lab on 7/1/14).
Methods: After practicing protist-identification with a pre-made mixture of protists provided in laboratory, 2 samples of water from the Hay Infusion Culture of Transect 2 were mounted on 2 separate slides for observation. It is important to note here the rather pungent odor of the Hay Infusion Culture, which smelled strongly of fecal matter and mold - common to stagnant bodies of water. There was no visible mold floating in the system, but a moldy scent was present before it was disturbed by a pipette to make the slides. The first sample was taken from the water near the top of the culture and the second sample was taken from water near the bottom of the culture. Using a dichotomous key of possible common protists that could be found in the mixture, 3 different varieties were identified and drawn for record.
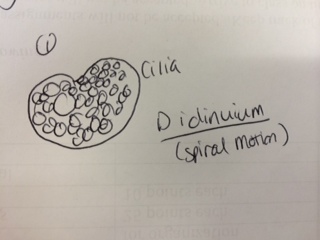
Results & Conclusions: In the water sample taken from the bottom of the Hay Infusion Culture, no protists were found to be identified under the microscope using the dichotomous key. However, 3 distinct protists were easily identified using the dichotomous key in the sample from the top of the Hay Infusion Culture. In the top "layer" of this culture, there was some plant material floating and gathered at the top. The 3 protists found there - Didinium, Paramecium, and Volovox - it is interesting to note that the Volovox does photosynthesize, which is confirmed by the presence of chloroplasts and a green coloring to the colonies found in the water sample. The presence of Volovox near the surface of the Hay Infusion water is expected, as it uses photosynthesis for energy and, thus, requires access to sunlight and oxygen at the top of the water. The presence of Didinium and Paramecium is particularly interesting as these often exhibit a predator-prey relationship, where Didinium is the predator and Paramecium is the prey. Finding them in the same sample is logical, as Didinium cannot exist without an energy source and Paramecium is one of the most common protists found in stagnant, fresh water. The Volovox colony found measured ~ 400 µm in size. 2 Didinium were found, one ~80 µm in size and the other slightly larger at ~120 µm in size. Several paramecium were identified at a size of ~ 200 µm each, suggesting either Paramecium multimicronucleatum or Paramecium caudatum (from the dichotomous key).
Representation illustrations of each protist are included below:
After identification of the protists, samples of the Hay Infusion Culture were created to plate serial dilutions. 4 sterile tubes with 10 mLs of sterile broth, each, were labeled with serial dilution factors, 10^-2, 10^-4, 10^-6, and 10^-8, respectively. To begin the serial dilution, 100 µL of Hay Infusion liquid was added to the 10^-2 sterile broth, capped, and swirled. Then, 100 µL of the 10^-2 solution was removed and added to the 10^-4 sterile broth, capped, and swirled for mixing. Then the process was repeated to create the 10^-6 and 10^-8 solutions, created from the previous larger magnitude in the series. Once all 4 serial dilutions were made, 100 µL of each was plated on labeled agar plates: 10^-3, 10^-3 T, 10^-5, 10^-5 T, 10^-7, 10^-7 T, 10^-9, 10^-T, where "T" denotes an agar solution with tetracycline antibiotic present to test for possible antibiotic resistance in future study. The 8 serial dilution plates were then placed agar-side-down in a plate rack in the biology lab with access to sunlight (near a clear window).
Predictions: Thinking about the Didinium present, the idea of the predator-prey relationship is once again notable for the ways in which this species meets its needs of life. It has an energy source (phagocytosis of the much larger Paramecium). It is able to move about its surroundings via cilia - which produced a spiral motion as observed under the microscope. As a protist, this organism can reproduce either sexually or asexually and this leads to increased survival in the environment as it does not require other Didinium present to increase in population. If the Hay Infusion were to go on for another 2 months, I predict that the Didinium species would still be in existence, so long as there was a food source for the Paramecium upon which the Didinium preys. Allowed to sit stagnant in an open container of water with other organisms and exchanges of energy (notably, photosynthesis) occurring around, there is a high possibility that the populations of these 3 protists would no only survive, but have increased in population size (though not exponentially). So long as there are resources for life, these protists can continue to live in the Hay Infusion Culture for another 2 months.
______________________________________________
Lab 3: Microbiology and Identifying Bacteria with DNA Sequence
7/8/14
Objectives: The objectives of this lab exploration were to understand the characteristics of bacteria and to observe the antibiotic resistance in the bacteria collected from the Hay Infusion Culture. Additionally, the objective was to begin PCR sequencing to further explore DNA of bacteria and how to use markers to identify species and species features.
Methods: To begin, the agar nutrient plates that were prepared on 7/3/14 were collected from the racks in the windows. Preliminary observation showed strong bacterial growth on each plate from the serial dilution series. Preliminary observation of the plates allowed for counting of the bacterial colonies, which are recorded in the table below:
Of the plates, 4 were selected for further, more in-depth study: 10^-7, 10^-9, 10^-7 Tetracycline, and 10^-9 Tetracycline. These were selected because the colonies were relatively separate from each other and observable with the naked eye and with microscopy. After the 4 plates were selected, 1 slide from each plate was prepared with a standard Gram stain procedure. While the slides were drawing, initial observations were made via dissecting microscope and recorded in Table 2 (seen below). Observations at this point were limited to size and shape of bacterial colonies as well as some characteristic observations (color, texture, etc.) After preliminary observations were made and recorded, the prepared Gram stain slides were studied in the compound microscope (at 4x, 10x, and 40x) and further observations were recorded as to the shape of the individual bacterium and their Gram positive or negative characteristics (as indicated by the stain). The observations of the 3 most clear slides were recorded in the table below:
After concluding observations, the bacterial growth plates were used in the first step of a PCR sequencing for 16s. Bacteria from each of the 3 plates observed above (Table 2) were boiled in 100µL of sterile water for 10 minutes. Then, the boiled samples were then centrifuged for 5 minutes at 13,400 rpm. 3 PCR tubes were prepared with 20 µL of PCR primer and mixed to dissolve the prepared PCR bead. Then 5µL of the supernatant from the centrifuged material was added to the PCR primer and placed in a thermocycler to await sequencing in an agarose gel. The primer was prepared as to sequence for the 16s gene, a procedure that will be completed on a future date.
Results & Discussion: The samples of water taken from the Hay Infusion Culture, as studied on 7/3/14, contained several protozoa and were suspected to contain several varieties of bacteria. However, it was not likely that any Archaea species would grow on the agar plates because these are typically found in extreme environments (places with very high salt content, very high temperatures, or other extremes). The Hay Infusion Culture was taken from a suburban area that is not an extreme environment, therefore it is not probable that any Archaea would be found on the plates. However, both types of plates (agar and agar + Tetracycline) did show growth of various bacteria. Before studying the bacteria, it is important to discuss the Hay Infusion Culture and the changes from the previous week. After 5 additional days in the lab, the smell of the culture grew stronger and smelled strongly of fecal matter, suggested a possible presence of E. coli or similar bacteria. A small amount of visible mold was present on the top. This change in appearance and scent is due to increased biotic activity in the sample and consumption of nutrients within the culture.
Turning back to the bacterial samples and plates, the different colony types that were visible suggested a variety of bacteria growing on the plates. A very noticeable difference between the agar plates and the agar + Tetracycline plates was the type of bacteria visible before microscopy. Upon initial observations, it is important to note how the Tetracycline had an obvious effect on the bacterial colony growth. On the plates without the presence of Tetracycline, there was obvious successful reproduction seen in the innumerable colonies forming lawns. On the highest dilution plate (10^-9) without the presence of Tetracycline, there were 163 colonies visible to count without the need of a microscope. The same dilution magnitude on a plate with Tetracycline added, however, did produce a visible reduction in bacterial colony number (18) and the colonies were visibly different - mostly orange in color. This is why orange bacterial colonies were selected for further study from the Tetracycline plates and white bacterial colonies were selected from the plates without Tetracycline.
The addition of the Tetracycline to the plates was done to "screen" for bacteria types which are resistant to the antibiotic - as Tetracycline is a common resistance in bacteria. By adding it to the dilution plates, it produced 2 distinct groups of bacteria to study and Gram stain. Eventually these bacteria will be identified from PCR sequencing. Tetracycline is an important and broad-spectrum antibiotic used in a variety of treatments in humans, including infections of the urinary tract, sexually transmitted diseases, and - more commonly - acne and other dermatological conditions (Dréno, et. al). The bacteria types sensitive to Tetracycline include mostly bacillus bacteria and the mechanism of action that the Tetracycline takes on the bacteria is to inhibit a binding site on the mRNA of the bacteria so that it is unable to successfully copy its genetic material and reproduce (IUPAC).
Returning to the consideration of the Gram slides made from these bacterial colonies (as described above), microscopy observations were made as to the shape and Gram positive or negative nature of the bacteria. Interestingly, the orange bacterial colonies present on the Tetracycline were found to be Gram-positive upon staining and the white colonies grown without the presence of Tetracycline were Gram-negative. Observations for the 3 most clear samples/slides were recorded in Table 2 (above).
Conclusions: The Hay Infusion Culture produced several varieties of bacteria, as evidenced by the variety of colonies on the dilution series agar plates.Initial observations, both of the colonies and then of the stained bacteria under microscopy, revealed a presence of 2 possible distinct varieties - white and orange - that will be identified in a future project with 16s sequencing with PCR. Site 2 produced a variety of protists (see Lab 2) and bacteria to study in a laboratory setting. Both the presence of the protists and bacteria are expected in such an environment but nonetheless interesting and worth further identification, especially of the different bacteria and the antibiotic-resistant variety(ies) found in Site 2.
Works cited: Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H (2004). "European recommendations on the use of oral antibiotics for acne". European Journal of Dermatology 14 (6): 391–9.
IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "tetracyclines".
Notes
- Place some notes here for visitors
- Example: This project is currently on hold until further notice.
|-
|colspan="2" style="background-color: #F2F2F2;"|
Recently Edited Notebook Pages
|}