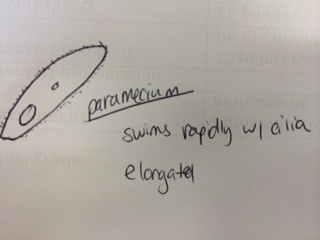User:Meredith Gleason1/Notebook/Biology 210 at AU
Lab 1: Biological Life at AU
Date: 7/1/14
Objective:This project focuses on a transect of land near the campus of American University in Washington, DC. While most would consider the campus to be an "urban environment", there is in fact quite a bit of green space available to analyze the ecosystems that exist on campus.
Methods/Observations: I traveled to the site of transect, Site #2, and made preliminary observations about the site. Site #2 is located on the grounds of the Wesley Theological Seminary, located just outside the northwestern boundaries of American University's campus. The transect of land, approximately 20x20 feet in size, is a wooded area with 6 pine trees with a dense area of ground cover below. Of the 6 trees, 3 are coniferous and 3 are deciduous. This transect is surrounded on all sides by grass but within the confines of the ~20x20 area of the trees, the ground is not covered with grass, but is instead covered with pine needles that have fallen and various short ground plants, like ivies and common weeds. An aerial view of the area is seen here:
And an eye-level photo is seen here:
At the site, an 11.6 g sample was taken, including soil found ~10 feet between two coniferous trees, along with pine needles and small leaves of ground ferns and ivies growing in the soil. Upon return to the lab, a Hay Infusion Culture was made with the entire soil sample, 0.1 g of dried milk, and 500 mL of filtered, Deerpark water in a large jar. The sample was vigorously mixed and left - with the lid open - on a lab bench to slowly separate.
Results & Conclusions: The result was, primarily, to make observations and set up a Hay Infusion Culture to be able to identify protists at a future date. Conclusions at this point are not appropriate as the infusion culture has not been studied.
______________________________________________
Lab 2: Identifying Algae and Protists
7/3/14
Objective: The objective of this laboratory investigation was to practice using the dichotomous key of possible protists to identify protists in a sample from the Hay Infusion Culture (produced in the previous lab on 7/1/14).
Methods: After practicing protist-identification with a pre-made mixture of protists provided in laboratory, 2 samples of water from the Hay Infusion Culture of Transect 2 were mounted on 2 separate slides for observation. It is important to note here the rather pungent odor of the Hay Infusion Culture, which smelled strongly of fecal matter and mold - common to stagnant bodies of water. There was no visible mold floating in the system, but a moldy scent was present before it was disturbed by a pipette to make the slides. The first sample was taken from the water near the top of the culture and the second sample was taken from water near the bottom of the culture. Using a dichotomous key of possible common protists that could be found in the mixture, 3 different varieties were identified and drawn for record.
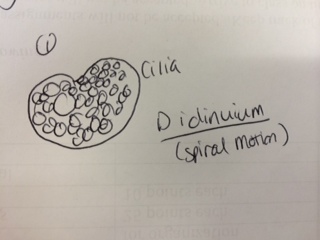
Results & Conclusions: In the water sample taken from the bottom of the Hay Infusion Culture, no protists were found to be identified under the microscope using the dichotomous key. However, 3 distinct protists were easily identified using the dichotomous key in the sample from the top of the Hay Infusion Culture. In the top "layer" of this culture, there was some plant material floating and gathered at the top. The 3 protists found there - Didinium, Paramecium, and Volovox - it is interesting to note that the Volovox does photosynthesize, which is confirmed by the presence of chloroplasts and a green coloring to the colonies found in the water sample. The presence of Volovox near the surface of the Hay Infusion water is expected, as it uses photosynthesis for energy and, thus, requires access to sunlight and oxygen at the top of the water. The presence of Didinium and Paramecium is particularly interesting as these often exhibit a predator-prey relationship, where Didinium is the predator and Paramecium is the prey. Finding them in the same sample is logical, as Didinium cannot exist without an energy source and Paramecium is one of the most common protists found in stagnant, fresh water. The Volovox colony found measured ~ 400 µm in size. 2 Didinium were found, one ~80 µm in size and the other slightly larger at ~120 µm in size. Several paramecium were identified at a size of ~ 200 µm each, suggesting either Paramecium multimicronucleatum or Paramecium caudatum (from the dichotomous key).
Representation illustrations of each protist are included below:
After identification of the protists, samples of the Hay Infusion Culture were created to plate serial dilutions. 4 sterile tubes with 10 mLs of sterile broth, each, were labeled with serial dilution factors, 10^-2, 10^-4, 10^-6, and 10^-8, respectively. To begin the serial dilution, 100 µL of Hay Infusion liquid was added to the 10^-2 sterile broth, capped, and swirled. Then, 100 µL of the 10^-2 solution was removed and added to the 10^-4 sterile broth, capped, and swirled for mixing. Then the process was repeated to create the 10^-6 and 10^-8 solutions, created from the previous larger magnitude in the series. Once all 4 serial dilutions were made, 100 µL of each was plated on labeled agar plates: 10^-3, 10^-3 T, 10^-5, 10^-5 T, 10^-7, 10^-7 T, 10^-9, 10^-T, where "T" denotes an agar solution with tetracycline antibiotic present to test for possible antibiotic resistance in future study. The 8 serial dilution plates were then placed agar-side-down in a plate rack in the biology lab with access to sunlight (near a clear window).
Predictions: Thinking about the Didinium present, the idea of the predator-prey relationship is once again notable for the ways in which this species meets its needs of life. It has an energy source (phagocytosis of the much larger Paramecium). It is able to move about its surroundings via cilia - which produced a spiral motion as observed under the microscope. As a protist, this organism can reproduce either sexually or asexually and this leads to increased survival in the environment as it does not require other Didinium present to increase in population. If the Hay Infusion were to go on for another 2 months, I predict that the Didinium species would still be in existence, so long as there was a food source for the Paramecium upon which the Didinium preys. Allowed to sit stagnant in an open container of water with other organisms and exchanges of energy (notably, photosynthesis) occurring around, there is a high possibility that the populations of these 3 protists would no only survive, but have increased in population size (though not exponentially). So long as there are resources for life, these protists can continue to live in the Hay Infusion Culture for another 2 months.
______________________________________________
Lab 3: Microbiology and Identifying Bacteria with DNA Sequence
7/8/14
Objectives: The objectives of this lab exploration were to understand the characteristics of bacteria and to observe the antibiotic resistance in the bacteria collected from the Hay Infusion Culture. Additionally, the objective was to begin PCR sequencing to further explore DNA of bacteria and how to use markers to identify species and species features.
Methods: To begin, the agar nutrient plates that were prepared on 7/3/14 were collected from the racks in the windows. Preliminary observation showed strong bacterial growth on each plate from the serial dilution series. Preliminary observation of the plates allowed for counting of the bacterial colonies, which are recorded in the table below:
Of the plates, 4 were selected for further, more in-depth study: 10^-7, 10^-9, 10^-7 Tetracycline, and 10^-9 Tetracycline. These were selected because the colonies were relatively separate from each other and observable with the naked eye and with microscopy. After the 4 plates were selected, 1 slide from each plate was prepared with a standard Gram stain procedure. While the slides were drawing, initial observations were made via dissecting microscope and recorded in Table 2 (seen below). Observations at this point were limited to size and shape of bacterial colonies as well as some characteristic observations (color, texture, etc.) After preliminary observations were made and recorded, the prepared Gram stain slides were studied in the compound microscope (at 4x, 10x, and 40x) and further observations were recorded as to the shape of the individual bacterium and their Gram positive or negative characteristics (as indicated by the stain). The observations of the 3 most clear slides were recorded in the table below:
After concluding observations, the bacterial growth plates were used in the first step of a PCR sequencing for 16s. Bacteria from each of the 3 plates observed above (Table 2) were boiled in 100µL of sterile water for 10 minutes. Then, the boiled samples were then centrifuged for 5 minutes at 13,400 rpm. 3 PCR tubes were prepared with 20 µL of PCR primer and mixed to dissolve the prepared PCR bead. Then 5µL of the supernatant from the centrifuged material was added to the PCR primer and placed in a thermocycler to await sequencing in an agarose gel. The primer was prepared as to sequence for the 16s gene, a procedure that will be completed on a future date.
Results & Discussion: The samples of water taken from the Hay Infusion Culture, as studied on 7/3/14, contained several protozoa and were suspected to contain several varieties of bacteria. However, it was not likely that any Archaea species would grow on the agar plates because these are typically found in extreme environments (places with very high salt content, very high temperatures, or other extremes). The Hay Infusion Culture was taken from a suburban area that is not an extreme environment, therefore it is not probable that any Archaea would be found on the plates. However, both types of plates (agar and agar + Tetracycline) did show growth of various bacteria. Before studying the bacteria, it is important to discuss the Hay Infusion Culture and the changes from the previous week. After 5 additional days in the lab, the smell of the culture grew stronger and smelled strongly of fecal matter, suggested a possible presence of E. coli or similar bacteria. A small amount of visible mold was present on the top. This change in appearance and scent is due to increased biotic activity in the sample and consumption of nutrients within the culture.
Turning back to the bacterial samples and plates, the different colony types that were visible suggested a variety of bacteria growing on the plates. A very noticeable difference between the agar plates and the agar + Tetracycline plates was the type of bacteria visible before microscopy. Upon initial observations, it is important to note how the Tetracycline had an obvious effect on the bacterial colony growth. On the plates without the presence of Tetracycline, there was obvious successful reproduction seen in the innumerable colonies forming lawns. On the highest dilution plate (10^-9) without the presence of Tetracycline, there were 163 colonies visible to count without the need of a microscope. The same dilution magnitude on a plate with Tetracycline added, however, did produce a visible reduction in bacterial colony number (18) and the colonies were visibly different - mostly orange in color. This is why orange bacterial colonies were selected for further study from the Tetracycline plates and white bacterial colonies were selected from the plates without Tetracycline.
The addition of the Tetracycline to the plates was done to "screen" for bacteria types which are resistant to the antibiotic - as Tetracycline is a common resistance in bacteria. By adding it to the dilution plates, it produced 2 distinct groups of bacteria to study and Gram stain. Eventually these bacteria will be identified from PCR sequencing. Tetracycline is an important and broad-spectrum antibiotic used in a variety of treatments in humans, including infections of the urinary tract, sexually transmitted diseases, and - more commonly - acne and other dermatological conditions (Dréno, et. al). The bacteria types sensitive to Tetracycline include mostly bacillus bacteria and the mechanism of action that the Tetracycline takes on the bacteria is to inhibit a binding site on the mRNA of the bacteria so that it is unable to successfully copy its genetic material and reproduce (IUPAC).
Returning to the consideration of the Gram slides made from these bacterial colonies (as described above), microscopy observations were made as to the shape and Gram positive or negative nature of the bacteria. Interestingly, the orange bacterial colonies present on the Tetracycline were found to be Gram-positive upon staining and the white colonies grown without the presence of Tetracycline were Gram-negative. Observations for the 3 most clear samples/slides were recorded in Table 2 (above).
Conclusions: The Hay Infusion Culture produced several varieties of bacteria, as evidenced by the variety of colonies on the dilution series agar plates.Initial observations, both of the colonies and then of the stained bacteria under microscopy, revealed a presence of 2 possible distinct varieties - white and orange - that will be identified in a future project with 16s sequencing with PCR. Site 2 produced a variety of protists (see Lab 2) and bacteria to study in a laboratory setting. Both the presence of the protists and bacteria are expected in such an environment but nonetheless interesting and worth further identification, especially of the different bacteria and the antibiotic-resistant variety(ies) found in Site 2.
Works cited: Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H (2004). "European recommendations on the use of oral antibiotics for acne". European Journal of Dermatology 14 (6): 391–9.
IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "tetracyclines".
______________________________________________
Lab 4: Plantae and Fungi
7/10/14
Objectives: The objectives of this project were to understand the characteristics and diversity of plants and to appreciate the function and importance of fungi. This was accomplished by a further study of the life found within Transect Site #2 at American University.
Methods: The project consisted of several different stages/steps, so please see each separate section below for a description of each particular stage.
1. After walking to Transect Site #2, located just outside of American University's northwest boundary and on the lawn of Wesley Theological Seminary, several plant samples were collected. The first (Sample A) consisted of leaf samples from 4 ground-cover plant species and 1 from a low-hanging pine branch from one of the site's 6 trees. The 5 samples are shown below in two pictures, along with a table of observations/descriptions:
Table 1:  Plant samples from Transect 2:
Plant samples from Transect 2:
Additionally, a sample of leaf litter (~500g) was collected from an area with soft soil and dead leaves under another tree at Transect 2. This sample (Sample B) was also brought back to the lab and used in a second stage.
2. Using Sample B (leaf litter from Transect 2), a Berlese Funnel was set up to collect invertebrates from the sample. Most of the sample collected was placed into a plastic funnel which was attached to a 50 mL tube containing 50% ethanol/50% water. The funnel and tube were sealed together with parafilm and this was attached to a ring stand to hold it. The ring stand with the funnel and leaf litter was transferred to a large, empty aquarium where it was placed under an incandescent bult (about 1-2 inches from the top of the leaf litter) and was covered with foil. A picture of the Berlese funnel is shown below for clarity. The results of the Berlese Funnel studying the leaf litter from Transect 2 will be used on a future date.
3. The third stage of the project's study included studying various plant and fungi species with the naked eye and under microscopy. A flower - a mature Lily - was deconstructed in order to study angiosperm reproductive structures. After that, a species of moss (Polytrichum) was observed under the moss, specifically looking at the antheridium and the archegonium. After observing the lily and the moss, several seeds were studied to determine monocot/dicot (see, "Results" below). After studying plant structures, attention was turned to fungi and the structures of the Rhizopus stolonifer (black bread mold) were studied under microscopy, where structures such as the hyphae and the rhizoids were identified growing in the nutrient agar. Below is included a drawing of observations made through the microscope.
4. The final procedure was to use the PCR samples from Lab 3 on 7/8/14 in a DNA electrophoresis study. 5 µL of PCR bacteria sample was loaded into an agarose gel - along with a ladder and marking dye. A preliminary picture of the electrophoresis result is included below, and further studies will be discussed on a future date.
Results/Discussion: In reference to Stage 1 (see "Methods", above), a description of observations - including about the vascularization of each - can be found in the included table, Table 1. From the image of Sample A, #3 is clearly a sample of pine needs from a low-hanging pine branch found in the transect. Samples 1-4 are more difficult to identify. According to a guide from the Chesapeake Bay Native Plant Center, plant #1 is possibly the False Solomon's Seal, which is often found in ground cover in the Washington, DC area. Plant #4, interestingly, is likely Downy Solomon's Seal, a related plant species. Plant #5 is likely an Arrowhead, a plant commonly found across the United States but especially in areas considered marshes, like Washington, DC. Plant #2 was not readily identified and at this point, it is not appropriate to venture a likely guess as to its identification. A seed (pine cone) was brought back from the Transect Site 2 (found in Sample B - leaf litter) however it was used in the Burlese Funnel procedure and was not dissected for further study as to its monocot or dicot nature.
In reference to Stage 2 - the construction of the Burlese Funnel - this step requires more time to make proper conclusions, but a preliminary conclusion would be the presence of several invertebrate species, especially common insects found in ground cover in the Mid-Atlantic region. The invertebrates collected from the Burlese Funnel, hopefully preserved in the ethanol solution below, will be studied on a further date for identification purposes.
In reference to Stage 3, the mold fungi studied was very interesting. In contrast to the plants described above, the black bread mold employs a different method of reproduction. The sporangia and rhizoids growing on the agar indicate reproduction, as molds/fungi frequently use spores to reproduce. See a hand-drawn picture of the sporangia/rhizoids complex in the Rhizopus stolonifer, above.
In reference to Stage 4, it is not appropriate to make conclusions about the DNA electrophoresis performed until further analysis can be performed on a future date.
(Source consulted: http://www.nativeplantcenter.net/guides/chesapeakenatives.pdf)
______________________________________________
7/15/14
Lab 5: Invertebrates
Objectives: The objectives of this project are to understand the importance of invertebrates and to apply understanding of how simple systems evolved into more complex systems through investigating invertebrates.
Methods/Observations:
I. Using a dissecting scope, Planaria was observed for movement and simplicity of structure. Next, a cross section of a Planaria was available to study under the scope. Unfortunately, all of the samples were dead so information concerning their movement (see, "Results") is based on previously-documented information. Next, cross sections of nematodes and Annelida were also observed to compare internal development and structure of their increasingly more developed body plans.
II. The Berlese Funnel set-up from 7/10/14 was broken down to collect the invertebrate specimens. After carefully removing the funnel with soil detritus from the top, the tube of ethanol contained some dirt and various invertebrates. The ethanol was poured into 2 separate petri dishes and moved to microscope stations for study under dissecting scope. Observations were made via dissecting scope and specific samples were transferred to depression slides in order to obtain measurements (in nm). Organisms found in the ethanol solution - invertebrates from Transect 2 - were identified and described (see Table 1, below).
III. Members of the lab group returned to Transect Site 2 for the third time and recorded observations about the types of vertebrates found at the site.
Results & Discussion: I. Invertebrates are an incredibly varied group of organisms, as evidenced by the types studied under the microscope and with the naked eye. Considering organisms like nematodes and flatworms (Planaria), it is possible to see how body structure developed different in these worms. The cross sections of the two reveal structural differences in the internal development of the gut. Nematodes have what is called a "pseudocoelum" - an incompletely lined body cavity and this acts as a primitive circulatory system. Planaria, however, is a less developed invertebrate, and it does not have a pseudocoelum. Rather, it is an "acoelomate", or an organism without a lined gut. Photos of the cross sections of each respective organism via microscopy are seen below:
Figure 1: Cross section of Planaria (flat worm), acoelomate.

Figure 2: Cross section of Nematode, pseudocoelomate.

Considering the development of each respective worm's gut, we can learn about the germ layers present in embryonic development and the development (or lack thereof) of muscle layers. Planaria, flat worms, are primitive worms with only 2 germ layers, which are responsible for the lack of a sophisticated gut and body structure. This also means that movement of these organisms is less refined, as compared to higher worms, and their movement is more creeping and less purposeful as we can see in other worms. There is no real evidence of a "muscle system" within the planaria, which is due to their 2 germ layers, the endoderm and ectoderm. The nematode, a round worm, is more developed than the planaria and does have all 3 germ layers in embryonic development (endoderm, ectoderm, and mesoderm) and this leads to a more developed gut and a primitive musculature which allows for more refined, purposeful movement. However, these worms have no appendages with which to aid in movement which is interesting when considering other invertebrates - notably insects - that have developed appendages through evolution.
There were many types of invertebrates present in the Berlese funnel sample taken from Transect 2. These invertebrates - primarily from Insecta - were preserved in the ethanol solution to allow for observation under the microscope. Below is a table with information on 5 organisms identified using the help of a dichotomous key and general information on different invertebrates. Additionally, there are photos of some of these organisms under microscopy.
Table 1: Identification of 5 invertebrates from Transect 2.

Image 4: Various invertebrates

Image 5: Various invertebrates including millipede (arthropoda) and ant (hymenoptera)

There were various sizes and types of invertebrates found in the leaf litter, especially arachnids and insects (arthropods). The largest organism identified in our sample was the millipede and there were 3 of them present. The smallest - and by far the most numerous present - were small ticks. Very small invertebrates were found within the leaf litter, suggesting that many, many more live in the area and are part of the food chain that exists there.
In addition to invertebrates, several invertebrate species are common on the American University Campus and have been spotted in or around Transect Site 2 area. (See Table 2, below). These common vertebrates interact with the Transect Site 2, using it as their home or as a food source is likely for these mammals and birds identified.
Table 2: Vertebrate Identification of Transect Site 2

These vertebrates all belong to the Animal Kingdom (Animalia), but have varying classifications. Scientific names are included above, and a more complete classification is found below:
1. White tail deer: Animalia Chordata Mammalia Artiodactyla Cervidae Capreolinae Odocoileus virginianus.
2.Eastern grey squirrel: Animalia Chordata Mammalia Rodentia Sciuridae Sciurus Sciurus carolinensis
3. Little brown bat: Animalia Chordata Mammalia Chiroptera Vespertilionidae Myotinae Myotis lucifugus
4. Blue jay: Animalia Chordata Aves Passeriformes Corvidae Cyanocitta cristata
5. Common grackle: Animalia Chordata Aves Passeriformes Icteridae Quiscalus quiscula
In the transect, the organisms detailed above are part of the larger ecosystem that is Transect 2. The trees found in the site likely serve as homes for many of the insects and certainly the birds. Additionally, the transect site is shady and protected in a way by the trees, which some of the organisms use as protection and/or temporary shelter. The insects are certainly a food source for the birds and the brown bat that inhabits the area. The vegetation discussed in Lab 4 is a source of food for the white tail deer. It is likely that the squirrel feeds on vegetation and on seeds from the trees or other plants in the transect. None of these species is carnivorous, but assuming there are some carnivores in nearby areas (Rock Creek Park), it is easy to imagine them as part of that food web as well, the larger vertebrates like birds and squirrels serving as food for carnivorous predators.
Pictured below is an artistic rendering of a possible food web for the organisms in Transect Site 2. The sun provides the energy for the plants to use for photosynthesis. The plants provide a food source for the invertebrates some some vertebrates. The trees and other plants also provide a habitat for the organisms. The invertebrates and vertebrates feed on the plants and their feces nourish the soil, so along with the continued presence of the sun and the nutrients available to the plants, the invertebrates and vertebrates have a food source. Also, the vertebrates can serve as a food source for carnivorous organisms.
Resources consulted: Smithsonian Vertebrate Zoology, online. http://www.mnh.si.edu/
Notes
- Place some notes here for visitors
- Example: This project is currently on hold until further notice.
|-
|colspan="2" style="background-color: #F2F2F2;"|
Recently Edited Notebook Pages
|}