User:Nouf Abuladel/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| (123 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Mar 22nd, 2014''' | '''''Zebrafish lab (embryology) ''''' | |||
[[INTRO:]] The purpose of this lab was to study the effects of different environment conditions on zebra fish embryonic development. Thus, we focused on examining the development stages of zebra fish embryos that were exposed to light. Light exposure can negatively affect the development of zebra fish embryos such as retinal physiology (Saszik & Bilotta, 1999). So, it's hypothesized that zebra fish embryos in an environment exposed to light will face some defects in their development stages. | |||
[[METHODS:]] We collected 40 healthy translucent zebra fish embryos and transferred them into two petri dishes labeled as (Control and Treatment "light"). Each dish plate had 50% of the 40 embryos. Since the independent variable is light, no extra substance was added other than water, for both plates. We recorded observation based on some dependent variables such as hatching, viability, movement, amount of yolk, eye pigmentation and movement, development of body and tail pigmentation, heart rate, and length of tail, body and eye. In order to take measurements, we used depression slides for microscopic observations. Also, on each day of collecting observations, we had to remove empty embryonic sacs and water and , then, replace that by x mL of water. On day 7, we fixed three samples from the control and light plates. We observed tail length, entire body length and eye diameter using the fixed samples, on day 14. | |||
[[OBSERVATIONS:]] | |||
[[Image:Screen_Shot_2014-03-22_at_2.59.59_PM.png]] | |||
Table 1 and 2 show the observations of day# 0, which is the day that we collected our zebrafish embryos on. All of the embryos were alive and unhatched. The embryos were at different stages. Also, all of the 21-hour-old embryos were able to move their eyes. Since they were inside the embryonic sac, we could not observe measurements of tail, body, and heart rate. | |||
[[Image:Screen_Shot_2014-03-22_at_3.35.33_PM.png]] | |||
[[Image:Screen_Shot_2014-03-22_at_3.47.32_PM.png]] | |||
On day# 5, we were not able to take measurements of heart rate, and tail/entire body length and eye diameter because there were no depression slides available. | |||
[[Image:Screen_Shot_2014-03-22_at_4.03.33_PM.png]] | |||
[[Image:Screen_Shot_2014-03-22_at_4.11.30_PM.png]] | |||
Day # 12: snow day so couldn't take observations. | |||
Day# 14: All the control and light zebra fishes were dead except for 1 from the control plate, That is due to a temperature change and inadequate supply of water. However, we collected some data from the fixed slides (table 8 and 9). | |||
[[Image:Screen_Shot_2014-03-22_at_4.40.03_PM.png]] | |||
[[CONCLUSION:]] Zebra fishes that are exposed to light are not negatively affected by that environment. So our hypothesis is refuted since we didn't observe any big difference between the control and light plates. However, we noticed some differences in terms of length. For instance, zebra fishes' tails in light are smaller than the control ones (table 8 and 9). Moreover, the movement of the individuals in light plate are a little faster than those in control. | |||
''Nouf Abuladel'' | |||
---- | |||
'''Mar 1st, 2014''' | '''''Mini notebook (transect paper)''''' | |||
[[INTRO:]] After spending a considerable amount of time in studying numerous organisms of our transect and sequencing DNA from PCR samples, which were made earlier, we are, now, able to identify the species of those organisms. That is by just having the DNA sequences and doing some research. Since both PCR samples of our transect (#3) have worked and sequenced, we are going to "blast" them and google some "blasted" information to learn more about the species and get better foundation for the transect paper. " Voluminous work but fun" :)) | |||
[[METHODS:]] Procedure was described previously in lab# 3 and 4. | |||
[[OBSERVATIONS:]] | |||
[[Image:Screen_Shot_2014-02-28_at_8.43.55_PM.png]] [[Image:Screen_Shot_2014-02-28_at_10.50.07_PM.png]] | |||
[[CONCLUSION:]] All in all, the observations from pervious labs and "blasted" information are rather similar. Blasting the DNA sequence, different species of a same domain of microorganism (bacteria) were found. However, data from petri dishes (in class) is gram-negative bacteria and rod-shaped as well as the googled data. So, we are, now, going to combine all five labs together with some research to build up a good base for our papers. | |||
''Nouf Abuladel'' | |||
---- | |||
'''Feb 28th, 2014''' | '''''lab# 5''''' | |||
[[INTRO:]] The reason behind this lab is to understand the complexity of the invertebrates. Invertebrates are diverse; thus, by the end of this lab, we should be able to identify the phylum of each invertebrate. Furthermore, the study of invertebrates allows us to dive inside them and observe the tissues (specialized cells) that made up the complex structures of these organisms. Berlese Funnel, which was prepared last week, will be used to collect data. | |||
[[METHODS:]] First step of the lab was to observe different slides of different invertebrates under the microscope. We examined planaria in terms of movement, digestive tract and complexity. 2nd, we looked at nematodes' movement and structure. Lastly, we observed annelida in terms of layers (muscles), size and movement. After that, we had a chance to observe 5 different organisms of arthropods and classify them using a sheet that was provided in the lab. Finally, we transferred the preservative solution from the berlese funnel into two petri dishes. Then, we had to examine the organisms using a dissecting microscope. | |||
[[OBSERVATIONS:]] Planaria moves slowly like a worm. It has digestive tract and nervous system. After observing nematodes under the microscope, we concluded that it has multiple organized layers. Also, it moves like a worm. It's thin and about 450um . For annelida, it moves like a worm,too; actually, it has a round stretching movement. It's composed of three germ layers so it's much more complex. | |||
[[Image:Screen_Shot_2014-02-28_at_3.34.06_PM.png]] [[Image:Screen_Shot_2014-02-28_at_4.15.53_PM.png]] [[Image:Rsz 11rsz img 0343.jpg]] [[Image:Rsz_1rsz_1img_0336.jpg]] | |||
[[Image:Screen_Shot_2014-02-28_at_5.18.12_PM.png]] The five arthropods are ,in order: Insects, centipedes, crustaceans, arachnids, and millipedes. (Table 1) | |||
We found three organisms from our transect solution and they are: small brownish beetle (length= 3 mm), small brown-yellow spider (length= 4 mm), and a very small reddish mite (length= 1 mm). Besides, we had to observe two more organisms from West VA.: a brown centipede with many body segments (length= 10 mm), and a brownish bee with wings and legs (length= 2 mm). (Table 2) | |||
[[Image:Rsz_screen_shot_2014-02-28_at_32404_pm.jpg]] | |||
[[Image:Screen_Shot_2014-02-28_at_6.14.46_PM.png]] <<< Food web of organisms in transect# 3 | |||
We, also, observed five kinds of vertebrates: mouse, bluebirds, squirrels, american robin, and oriole. These organisms interact with biotic and abiotic factors within the transect to make up a continuous ecosystem. For example, mites, centipedes and other bugs might be the food for bluebirds and oriole. Plants (seeds, fruits and grains) are the food for mouse, squirrels and birds. Since millipedes and other bugs find their food in soil, bluebirds mouse and oriole are present there to eat them. Furthermore, the transect location is sunny so plants are able to grow and make their food via photosynthesis. Thus, squirrels and mouse are seen there to eat the seeds and fruits of the plants. Actually, trees are the places where mouse and squirrels hide under during cold weather. | |||
[[CONCLUSION:]] All in all, this lab was fun and easy to understand. The materials we observed were really beneficial for us to learn about the different organisms around AU environment. Actually, the organisms and micro organisms we found in our transect are amazingly diverse. However, we had to use samples of organisms from West VA. From this lab, the interaction between biotic and abiotic organisms in that transect is simply perfect and makes up such a small system (niche) in the transect (see food web). | |||
''Nouf Abuladel'' | |||
---- | |||
'''Feb 27th, 2014''' | '''''lab# 4''''' | |||
[[INTRO:]] The purpose of this lab is to identify the characteristics of both fungi and plantae. That is by observing several plant samples from our transect and examining them in terms of three features such as vascularization, structures and reproduction. Moreover, this lab will allow us to learn more about the structure of fungi. For the upcoming lab, we will set up the Berlese Funnel. | |||
[[METHODS:]] The very first step in that lab was running the PCR products by applying them into the agarose gel and choosing the best DNA samples to send them for sequencing. We were lucky to have our two samples (10^-3, and T-10^-3) worked perfectly in the gel. | |||
[[Image:Rsz_1rsz_img_0214.jpg]] | |||
After that, we went to collect some samples from our transect. We were asked to collect in plastic bags five different types of plant, seeds/flowers, and a bag full of wet soil. We identified the group of each of the five plants by using a tool that was provided for us in the lab. We, also, had to state the location, description, vascularization, characteristics and seeds/ flower of each sample of the plants.For the vascularization part, we had to examine that feature for each of the under the microscope and that is by making a cross section of each plant's stem. Additionally, we had a chance to look at and observe different parts of lily flower. So, we Identified the seeds/flower from our transect as either monocot or dicot. To observe fungi, we looked at fungal organisms from agar plates using both microscope and dissection one. | |||
In preparation for the next lab, we were asked to set up the berlese funnel by pouring about 25 ml of 50:50 ethanol/water solution into a bottle, a piece of screening material was taped inside the funnel, the funnel neck, then, was placed above the ethanol-water solution, and it held by a ring stand. Finally, the bag of soil, which was collected from the transect, was poured into the funnel. | |||
[[Image:Rsz_1rsz_img_0210.jpg]] | |||
The funnel was incubated under a lighted 40 watt lamp and covered with foil for a week. | |||
[[OBSERVATIONS:]] | |||
[[Image:Jjj.jpg]] [[Image:Screen_Shot_2014-02-27_at_6.56.23_PM.png]] [[Image: Transect_mapping.jpg]] | |||
'''(Table 1)''' Our transect's plants were diverse in terms of vascularization, shape and genus (also, see the map of our transect). There was no evidence of reproductive parts and that might be because of the weather(season). | |||
[[Image:Rsz_1rsz_img_0212.jpg]] The sample of fungi we observed was zygomycota. This type of fungi has spores, mycelium and sporangia. | |||
[[CONCLUSION:]] All in all, this lab was useful to learn about plants and fungi. It shows the diversity of organisms around AU environment and how these organisms and microorganisms diverse depending on the climate. Furthermore, we will be able to get familiar characterizing the organisms's species from the DNA samples after sequencing it. | |||
''Nouf Abuladel'' | |||
---- | |||
'''Feb 16th, 2014''' | '''''lab# 3''''' | '''Feb 16th, 2014''' | '''''lab# 3''''' | ||
[[INTRO:]] After observing several types of micro organisms within our transect and incubating the plates for a week, it is the time to look at bacteria and understand its characteristics. The further we study bacteria the more we get to learn about its cells and be able to distinguish between gram positive and gram negative. That would, also, help in examining the antibiotic resistance and | [[INTRO:]] After observing several types of micro organisms within our transect and incubating the plates for a week, it is the time to look at bacteria and understand its characteristics. The further we study bacteria the more we get to learn about its cells and be able to distinguish between gram positive and gram negative. That would, also, help in examining the antibiotic resistance and observing its affects on bacteria. Additionally,we'll be sequencing DNA from our Hay Infusion in preparation for next lab. | ||
[[METHODS:]] The very first procedure of this lab was to examine nutrient agar and tetracycline plates. We were asked to count the total number of colonies on each plate. | [[METHODS:]] The very first procedure of this lab was to examine nutrient agar and tetracycline plates. We were asked to count the total number of colonies on each plate. | ||
| Line 7: | Line 122: | ||
[[Image:Rsz_screen_shot_2014-02-16_at_31101_am.jpg]] | [[Image:Rsz_screen_shot_2014-02-16_at_31101_am.jpg]] | ||
Then , we chose three plates that we wanted to study their colonies. We labeled a place of interest on each of the three plates, so, we have labeled them as (10^-3, N three guys), (10^-3, T) and ( 10^-5, N). | Then, we chose three plates that we wanted to study their colonies. We labeled a place of interest on each of the three plates, so, we have labeled them as (10^-3, N three guys), (10^-3, T) and ( 10^-5, N). The identifications are based on colony description in terms of color, shape and texture, number of colonies, cell description in terms of shape and arrangement, and whether it's gram positive or negative. In order to see those micro organisms closely, we observe them under the microscope after we've spread a sample of the place, which we labeled on the plates, on a slide. Also,the slides were heated with the bacterial smear side up and,then, we coated the smear with crystal violet for a minute and rinsed it off with water. Next, the smear was covered with iodine for a minute and rinsed gently after that, moreover, the smear was decolorized with alcohol for 20 seconds and , then, covered with safranin for 25 seconds and rinsed gently again. Finally, the slides were dried from excess water carefully with paper towel. | ||
[[Image:Rsz_1screen_shot_2014-02-16_at_40512_am.jpg]] [[Image:IMG_0422.jpg]] | [[Image:Rsz_1screen_shot_2014-02-16_at_40512_am.jpg]] [[Image:IMG_0422.jpg]] | ||
| Line 13: | Line 128: | ||
The very last step was to prepare PCR for DNA sequence Identification. That's by choosing one of the plates that has the best characteristics to examine. A single colony of bacteria was added into 100 ul of water in a tube and it was incubated at 100 degrees celsius for 10 min. That tube, later on, was centrifuged. 5 ul of the supernatant was used for PCR. | The very last step was to prepare PCR for DNA sequence Identification. That's by choosing one of the plates that has the best characteristics to examine. A single colony of bacteria was added into 100 ul of water in a tube and it was incubated at 100 degrees celsius for 10 min. That tube, later on, was centrifuged. 5 ul of the supernatant was used for PCR. | ||
[[OBSERVATIONS:]] The smell and appearance of the Hay Infusion keep changing from week to week and even its concentration seems to be increased over the days. That is due to the fact that some of some microorganisms in the jar might have died or grown and, in fact, the bad smell | [[OBSERVATIONS:]] The smell and appearance of the Hay Infusion keep changing from week to week and even its concentration seems to be increased over the days. That is due to the fact that some of some microorganisms in the jar might have died or grown and, in fact, the bad smell comes from the growth of bacteria. The plates with antibiotic had less bacterial colonies while the plates without antibiotic had more colonies. So the effect of tetracycline on the number and the type of bacteria was definite and that shows that the antibiotic was effectively working. Tetracycline works by interfering with the ability of bacteria to produce proteins that are essential to them. Without these proteins, bacteria cannot grow. Moreover, after observing our slides we had two gram positive cells and one gram negative cell. | ||
[[CONCLUSION:]] Summing up, this lab was helpful in understanding bacteria, observing its cells more closely and learning about the antibiotic's affects on bacterial colonies' color, type and numbers. So, now we have considerable amount of information about the bacteria of our transect. Moreover, in this lab we made PCR sample of our Hay Infusion so that for next week we'll be able to run the PCR products on an agarose gel if the DNA looks good. Thus, we are going to analyze the DNA sequences from our transect. | |||
''Nouf Abuladel'' | |||
---- | ---- | ||
| Line 35: | Line 153: | ||
Nouf Abuladel | ''Nouf Abuladel'' | ||
| Line 70: | Line 188: | ||
Nouf Abuladel | ''Nouf Abuladel'' | ||
---- | ---- | ||
| Line 78: | Line 196: | ||
successfully entered text and username | successfully entered text and username | ||
Nouf Abuladel | ''Nouf Abuladel'' | ||
Latest revision as of 01:05, 25 March 2014
Mar 22nd, 2014 | Zebrafish lab (embryology)
INTRO: The purpose of this lab was to study the effects of different environment conditions on zebra fish embryonic development. Thus, we focused on examining the development stages of zebra fish embryos that were exposed to light. Light exposure can negatively affect the development of zebra fish embryos such as retinal physiology (Saszik & Bilotta, 1999). So, it's hypothesized that zebra fish embryos in an environment exposed to light will face some defects in their development stages.
METHODS: We collected 40 healthy translucent zebra fish embryos and transferred them into two petri dishes labeled as (Control and Treatment "light"). Each dish plate had 50% of the 40 embryos. Since the independent variable is light, no extra substance was added other than water, for both plates. We recorded observation based on some dependent variables such as hatching, viability, movement, amount of yolk, eye pigmentation and movement, development of body and tail pigmentation, heart rate, and length of tail, body and eye. In order to take measurements, we used depression slides for microscopic observations. Also, on each day of collecting observations, we had to remove empty embryonic sacs and water and , then, replace that by x mL of water. On day 7, we fixed three samples from the control and light plates. We observed tail length, entire body length and eye diameter using the fixed samples, on day 14.
Table 1 and 2 show the observations of day# 0, which is the day that we collected our zebrafish embryos on. All of the embryos were alive and unhatched. The embryos were at different stages. Also, all of the 21-hour-old embryos were able to move their eyes. Since they were inside the embryonic sac, we could not observe measurements of tail, body, and heart rate.
On day# 5, we were not able to take measurements of heart rate, and tail/entire body length and eye diameter because there were no depression slides available.
Day # 12: snow day so couldn't take observations.
Day# 14: All the control and light zebra fishes were dead except for 1 from the control plate, That is due to a temperature change and inadequate supply of water. However, we collected some data from the fixed slides (table 8 and 9).
CONCLUSION: Zebra fishes that are exposed to light are not negatively affected by that environment. So our hypothesis is refuted since we didn't observe any big difference between the control and light plates. However, we noticed some differences in terms of length. For instance, zebra fishes' tails in light are smaller than the control ones (table 8 and 9). Moreover, the movement of the individuals in light plate are a little faster than those in control.
Nouf Abuladel
Mar 1st, 2014 | Mini notebook (transect paper)
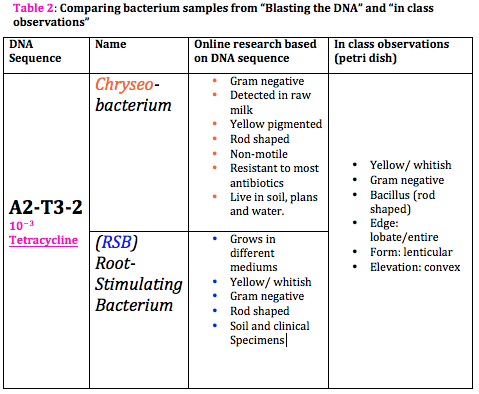
INTRO: After spending a considerable amount of time in studying numerous organisms of our transect and sequencing DNA from PCR samples, which were made earlier, we are, now, able to identify the species of those organisms. That is by just having the DNA sequences and doing some research. Since both PCR samples of our transect (#3) have worked and sequenced, we are going to "blast" them and google some "blasted" information to learn more about the species and get better foundation for the transect paper. " Voluminous work but fun" :))
METHODS: Procedure was described previously in lab# 3 and 4.
CONCLUSION: All in all, the observations from pervious labs and "blasted" information are rather similar. Blasting the DNA sequence, different species of a same domain of microorganism (bacteria) were found. However, data from petri dishes (in class) is gram-negative bacteria and rod-shaped as well as the googled data. So, we are, now, going to combine all five labs together with some research to build up a good base for our papers.
Nouf Abuladel
Feb 28th, 2014 | lab# 5
INTRO: The reason behind this lab is to understand the complexity of the invertebrates. Invertebrates are diverse; thus, by the end of this lab, we should be able to identify the phylum of each invertebrate. Furthermore, the study of invertebrates allows us to dive inside them and observe the tissues (specialized cells) that made up the complex structures of these organisms. Berlese Funnel, which was prepared last week, will be used to collect data.
METHODS: First step of the lab was to observe different slides of different invertebrates under the microscope. We examined planaria in terms of movement, digestive tract and complexity. 2nd, we looked at nematodes' movement and structure. Lastly, we observed annelida in terms of layers (muscles), size and movement. After that, we had a chance to observe 5 different organisms of arthropods and classify them using a sheet that was provided in the lab. Finally, we transferred the preservative solution from the berlese funnel into two petri dishes. Then, we had to examine the organisms using a dissecting microscope.
OBSERVATIONS: Planaria moves slowly like a worm. It has digestive tract and nervous system. After observing nematodes under the microscope, we concluded that it has multiple organized layers. Also, it moves like a worm. It's thin and about 450um . For annelida, it moves like a worm,too; actually, it has a round stretching movement. It's composed of three germ layers so it's much more complex.
 The five arthropods are ,in order: Insects, centipedes, crustaceans, arachnids, and millipedes. (Table 1)
The five arthropods are ,in order: Insects, centipedes, crustaceans, arachnids, and millipedes. (Table 1)
We found three organisms from our transect solution and they are: small brownish beetle (length= 3 mm), small brown-yellow spider (length= 4 mm), and a very small reddish mite (length= 1 mm). Besides, we had to observe two more organisms from West VA.: a brown centipede with many body segments (length= 10 mm), and a brownish bee with wings and legs (length= 2 mm). (Table 2)
 <<< Food web of organisms in transect# 3
<<< Food web of organisms in transect# 3
We, also, observed five kinds of vertebrates: mouse, bluebirds, squirrels, american robin, and oriole. These organisms interact with biotic and abiotic factors within the transect to make up a continuous ecosystem. For example, mites, centipedes and other bugs might be the food for bluebirds and oriole. Plants (seeds, fruits and grains) are the food for mouse, squirrels and birds. Since millipedes and other bugs find their food in soil, bluebirds mouse and oriole are present there to eat them. Furthermore, the transect location is sunny so plants are able to grow and make their food via photosynthesis. Thus, squirrels and mouse are seen there to eat the seeds and fruits of the plants. Actually, trees are the places where mouse and squirrels hide under during cold weather.
CONCLUSION: All in all, this lab was fun and easy to understand. The materials we observed were really beneficial for us to learn about the different organisms around AU environment. Actually, the organisms and micro organisms we found in our transect are amazingly diverse. However, we had to use samples of organisms from West VA. From this lab, the interaction between biotic and abiotic organisms in that transect is simply perfect and makes up such a small system (niche) in the transect (see food web).
Nouf Abuladel
Feb 27th, 2014 | lab# 4
INTRO: The purpose of this lab is to identify the characteristics of both fungi and plantae. That is by observing several plant samples from our transect and examining them in terms of three features such as vascularization, structures and reproduction. Moreover, this lab will allow us to learn more about the structure of fungi. For the upcoming lab, we will set up the Berlese Funnel.
METHODS: The very first step in that lab was running the PCR products by applying them into the agarose gel and choosing the best DNA samples to send them for sequencing. We were lucky to have our two samples (10^-3, and T-10^-3) worked perfectly in the gel.
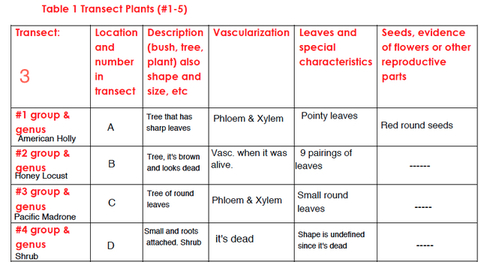
After that, we went to collect some samples from our transect. We were asked to collect in plastic bags five different types of plant, seeds/flowers, and a bag full of wet soil. We identified the group of each of the five plants by using a tool that was provided for us in the lab. We, also, had to state the location, description, vascularization, characteristics and seeds/ flower of each sample of the plants.For the vascularization part, we had to examine that feature for each of the under the microscope and that is by making a cross section of each plant's stem. Additionally, we had a chance to look at and observe different parts of lily flower. So, we Identified the seeds/flower from our transect as either monocot or dicot. To observe fungi, we looked at fungal organisms from agar plates using both microscope and dissection one.
In preparation for the next lab, we were asked to set up the berlese funnel by pouring about 25 ml of 50:50 ethanol/water solution into a bottle, a piece of screening material was taped inside the funnel, the funnel neck, then, was placed above the ethanol-water solution, and it held by a ring stand. Finally, the bag of soil, which was collected from the transect, was poured into the funnel.
The funnel was incubated under a lighted 40 watt lamp and covered with foil for a week.
(Table 1) Our transect's plants were diverse in terms of vascularization, shape and genus (also, see the map of our transect). There was no evidence of reproductive parts and that might be because of the weather(season).
 The sample of fungi we observed was zygomycota. This type of fungi has spores, mycelium and sporangia.
The sample of fungi we observed was zygomycota. This type of fungi has spores, mycelium and sporangia.
CONCLUSION: All in all, this lab was useful to learn about plants and fungi. It shows the diversity of organisms around AU environment and how these organisms and microorganisms diverse depending on the climate. Furthermore, we will be able to get familiar characterizing the organisms's species from the DNA samples after sequencing it.
Nouf Abuladel
Feb 16th, 2014 | lab# 3
INTRO: After observing several types of micro organisms within our transect and incubating the plates for a week, it is the time to look at bacteria and understand its characteristics. The further we study bacteria the more we get to learn about its cells and be able to distinguish between gram positive and gram negative. That would, also, help in examining the antibiotic resistance and observing its affects on bacteria. Additionally,we'll be sequencing DNA from our Hay Infusion in preparation for next lab.
METHODS: The very first procedure of this lab was to examine nutrient agar and tetracycline plates. We were asked to count the total number of colonies on each plate.
Then, we chose three plates that we wanted to study their colonies. We labeled a place of interest on each of the three plates, so, we have labeled them as (10^-3, N three guys), (10^-3, T) and ( 10^-5, N). The identifications are based on colony description in terms of color, shape and texture, number of colonies, cell description in terms of shape and arrangement, and whether it's gram positive or negative. In order to see those micro organisms closely, we observe them under the microscope after we've spread a sample of the place, which we labeled on the plates, on a slide. Also,the slides were heated with the bacterial smear side up and,then, we coated the smear with crystal violet for a minute and rinsed it off with water. Next, the smear was covered with iodine for a minute and rinsed gently after that, moreover, the smear was decolorized with alcohol for 20 seconds and , then, covered with safranin for 25 seconds and rinsed gently again. Finally, the slides were dried from excess water carefully with paper towel.
The very last step was to prepare PCR for DNA sequence Identification. That's by choosing one of the plates that has the best characteristics to examine. A single colony of bacteria was added into 100 ul of water in a tube and it was incubated at 100 degrees celsius for 10 min. That tube, later on, was centrifuged. 5 ul of the supernatant was used for PCR.
OBSERVATIONS: The smell and appearance of the Hay Infusion keep changing from week to week and even its concentration seems to be increased over the days. That is due to the fact that some of some microorganisms in the jar might have died or grown and, in fact, the bad smell comes from the growth of bacteria. The plates with antibiotic had less bacterial colonies while the plates without antibiotic had more colonies. So the effect of tetracycline on the number and the type of bacteria was definite and that shows that the antibiotic was effectively working. Tetracycline works by interfering with the ability of bacteria to produce proteins that are essential to them. Without these proteins, bacteria cannot grow. Moreover, after observing our slides we had two gram positive cells and one gram negative cell.
CONCLUSION: Summing up, this lab was helpful in understanding bacteria, observing its cells more closely and learning about the antibiotic's affects on bacterial colonies' color, type and numbers. So, now we have considerable amount of information about the bacteria of our transect. Moreover, in this lab we made PCR sample of our Hay Infusion so that for next week we'll be able to run the PCR products on an agarose gel if the DNA looks good. Thus, we are going to analyze the DNA sequences from our transect.
Nouf Abuladel
Feb 9th, 2014 | lab# 2
INTRO: This lab is all about identifying algae and protists. The identifications are based on the morphological characteristics such as shape, size and motility. Thus, we're using a tool called a "Dichotomous Key" to make the work easier in identifying the group of organisms from our Hay Infusion.
METHODS: Before we use our Hay Infusion, we looked at known samples of organisms, such as Gonium, under the microscope and that is just to get practiced and familiar with the tool "dichotomous key". After that, we divided the work on our group so that each individual observes a specific niche, such as, surface or near-vegetation niche. My part was to identify the organisms that are in the bottom of the jar.
Lastly, in order to prepare for lab# 3, we obtained four 10-ml-sterile-broth tubes to prepare the dilutions. 100 micro liters of the Hay Infusion was added to the first tube (10^-2). Also, we took 100 micro liters from the first tube and added it to the second tube. The process were repeated for the third and fourth tubes. Next, we obtained four nutrient agar plates labeled -3, -5,-7 and -9, additionally, three tetracycline plates labeled T-3, T-5, and T-7. we took 100 ul from the first tube and pour it into the plate (-3). By using a spreader, the 100-ul of the dilution was spread in the plate. [Exact procedure was repeated for the rest of the plates]. These plates will be incubated at room temperature over the next week.
OBSERVATIONS: Hay Infusion smells awful, some mold appeared on its surface and no green shoots. Also, the concentration of the Hay infusion has decreased. That's because some the organisms in the system died. Temperature and light are some the selective pressures that affected the compositions of the samples. Since we observed different niche from our transect, there were many different organisms such as two of Euglena (size=7 um), Stentor (size= 10 um), Paramecium (size= 80 um), Actinosphaerium (size= 50 um) and Gonium (size= 65 um).
CONCLUSION: All in all, we, now, know how to use dichotomous key to identify different micro organisms. also, we further understand how selective pressures affect a particular system and can lead to evolution. the plating procedure will, also, be helpful to understand the characteristics of bacteria in the upcoming lab.
Nouf Abuladel
2/6/14, lab 1 notes
Great work! Some notes: -Include subject headers such as "introduction", "methods", etc
-Make sure pics are included by Sunday
-Start working on building a map of your transect to detail your land and where your samples are taken from. We will talk about this more Wednesday
Good job! AP
Jan 31st, 2014 | lab# 1
INTRO: The purpose of this lab is to understand the meaning of the neutral selection and evolution. From those concepts, organisms that live in a niche (biotic and abiotic) can be observed through the lab. Also, we can see how these organisms change their behaviors from time to time and from place to place. So, now, we can look at our transect and be able to identify different organisms.
METHODS: Prior to preparing our transects, we had a chance to look at different types of algae by using the microscope: Chlamydomonas, Gonium, and Volvox. Thus, we identify the size, number of cells, reproductive type and functions.
Then, the transect was prepared from a place that has vegetation around it near Bender Arena which can help in preparing the Hay Fusion. That is by taking some of the vegetation and putting it in in a sterile 50ml conical tube. The Hay Fusion 500ml of water was added, 0.1 gram of powdered milk, in addition to the contents of our conical. After that, the Hay Fusion content was transferred into a jar.
OBSERVATIONS: Plants, bird and shrubs were our biotic. Abiotic factors that was observed were wet mulch, soil, sunlight, cigarette buts, rocks, a sprinkler,and a coca cola paper cup.
CONCLUSION: All in all, this lab will help us in studying microorganisms in the transect and in upcoming labs. Also, definitely, there will be a diverse of organisms that might be living in the surface and the bottom of the jar.
Nouf Abuladel
Jan 22nd, 2014 successfully entered text and username
Nouf Abuladel