User:Rebeka Winkler/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| (18 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Zebrafish Development in Retinoic Acid | |||
March 17, 2015''' | |||
Purpose: | |||
Comparing zebrafish, in embryonic stages and beyond, under conditions containing retinoic acid and conditions without reintoic acid. | |||
Introduction: | |||
Retinoic Acid is a metabolite of Vitamin A that is absolutely crucial to the normal development of chordate, high functioning, mammals. Retinoic acid functions as a stabilizer for Vitamin A in growth and development (Holland). Without enough retinoic acid or too much, mutations occur. In humans, too much retinoic acid can block the retinoic acid receptor resulting in predisposal to myeloid and lymphoid leukemia (Parrado et al.). In Zebra fish, an excess of retinoic acid can lead to mutations including a short or crooked tail, delayed hatching, ill formed or unformed valves on the heart and brain, and the absence of one or both eyes (Herrman). | |||
There are a number of reasons why zebra fish are used to study development. Their embryos are clear and development in the embryo is rapid compared to other species. Experimental variables can be injected directly into the water as zebra fish, in early developmental stages, accept their aquatic environment directly into their bloodstream (Institute of Molecular and Cell Biology). | |||
Materials and Methods: | |||
20 zebrafish eggs were placed in two separate dishes. In one dish, the control group, the zebrafish were placed in 10 Ml of water. In the other dish, the zebrafish were placed in 10 Ml of water and 5 Ml of retinoic acid. Every few days, the fish are fed a small amount of paramecium. Each week, the fish will be observed in order to measure size of fish, physical appearance of fish, and movement of fish all compared to the other group. On day seven of observation, three fish from each group was placed in preservative to keep them fixed in that stage of development. | |||
Results: | |||
Day One (2/20): | |||
Four days after their initial placement, there was no hatching in either of the dishes filled with embryos. Though three of the embryos were dead in the acid, as indicated by the collection of a mold looking substance and the embryo's grey color. One embryo was dead in the control dish of just water. | |||
Day Two (2/23): | |||
Three more fish in the acid had died equalling a total of 16 fish left in the acid group. One more had died in the control equalling 18 total fish in the control group. Most of the fish had hatched. | |||
Day Three (2/26): | |||
Three more dead in acid. One more dead in control. All fish are very active. Retinoic is faster movement but is more delayed to start moving. Hatched fish in acid are more jittery in movement compared to the gliding movement of the control. | |||
Day Four (3/6): | |||
On the final day of observation, all were dead in acid. Nine had reached full form in comparison to the control group. Their pigment was black and dark blue with a cloudy complexion.There was no motility. Six more are dead in the control, nine fish are alive with normal movement. They are 5 cm in length, larger than the fish in retinoic acid. | |||
Conclusions: | |||
Conclusions are drawn mostly from the day seven, fixed fish. Control fish are more structured, there is clear development organ development in a structured pattern. On 4x on the microscope fish are 15.5-17 mm. The back fin has very little development but structure is starting to form and there is no front fin development. The eye is 5x5.5 and there the pupil is transparent. The fixed fish on day 7 of the retinoic acid is 14 mm on 4x and has a grey pigment. Both back and side fins are starting to develop. Eye is 5x5.5 with no pupil. | |||
The embryos hatched normally in both groups, in a matter of 5-6 days though the ratio of fish hatched in the water without retinoic acid is higher. Upon basic observation of the fish there is no major difference between the two groups. The retinoic acid has proven it's tested effects in the case of zebrafish embryos. Though,the retinoic acid has proven it's tested effects in the case of zebrafish embryos. Organ development is disrupted in fish exposed to retinoic acid. There is a solid color on the zebrafish in the control group while the color on the retinoic acid fish is less blotchy. The heart and brain are not in the same place in the retinoic acid fish as in the control group meaning that there are unformed or malformed valves of the heart and brain. Eyes are also a little larger in the fish exposed to retinoic acid. There is also marginal higher death rate in the retinoic acid fish. Too much exposure to retinoic acid does result serious birth defects. | |||
References: | |||
Herrman, K. (1994). Teratogenic effects of retinoic acid and related substances on | |||
the early development of the zebra fish as assessed by a novel scoring system. Pergamon. 9.(3). 267-283. | |||
Holland, L. Z. (2007). "Developmental biology: A chordate with a | |||
difference". Nature 447 (7141): 153–155. | |||
Parrado, A., C. Chomienne, and R. A. Padua. (2000). Retinoic acid receptor alpha | |||
(RAR alpha) mutations in human leukemia. Leuk. Lymphoma 39:271-282. | |||
Institute of Molecular and Cell Biology. (2012). Zebrafish facility. A*Star Singapore. | |||
'''BW''' | |||
'''Identifying Bacteria | |||
March 2nd, 2105''' | |||
Purpose: | |||
Identifying bacteria | |||
Introduction: | |||
In previous labs we grew bacteria on agar plates with an without Tetracycline. After selecting a large, interesting sample the bacteria underwent PCR and was then placed on a gel electrophoresis. The PCR was then sent to an outside lab for sequencing specifically of the 16s gene. | |||
Materials and Methods: | |||
The website genewiz.com was used to seuence the 16S PCR product from the bacteria specific to each group.Then the BLAST software was used to identify and classify each sequence to a bacteria. | |||
Results: | |||
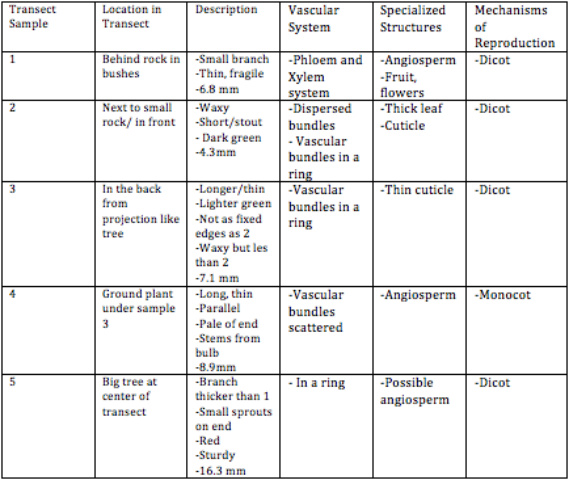
The original gel electrophoresis, shown in figure one, displayed the most successful PCR reaction in row two which was the sample chosen for sequencing. | |||
Figure 1: [[Image:FotoFlexer_circled.jpg]] | |||
After sequecning the rat data of the 16S PCR was: | |||
NNNNNNNNNGNNTNNAATGCANNCGANANGGAGCANNANTTGTCTGGGGGAGNGCGACGGGNGAGTNTATATCGGAACGT | |||
ACCCTANGTGGGGGATATCGTCTCGAAAGTTACGCTAATACCGCATACGATCTAAGGTGAAAGCGGGGGATCGCAAGACC | |||
TCGCGCTCGTGGAGCGGCCGATATCTGATTATATAGTTGGTANGATAAAAGCCTACCAAGGCGTCGATCTGTAGCTGGTC | |||
TGAGAGGACGACCAGCCACACTGGGACTGAGACACGGNCNAGACTCCTACGGGAGGCAGCAGTGGGGAATTTTGNACAAT | |||
GGGCGAAAGCCTGATCCANCAATGCCGCGTGNNNNNAGAAGGCCTTCGGGTTGTAAANCTCTTTTNTNNGGCNANGANAA | |||
GGNNGANAGCTAATANCCCNNTGGTTNTTGACGNTACCTNNAGAATAAGCACCGGCTNNNNTACTNTGNNANCAGCCGCG | |||
GATAATACNNNAGGNGTGCTNANNNGNNTNNTNNGGNNATNNANNTGNNGNNGTNANNNNNNNNGCCGCNNNGNNNGGGG | |||
TNTNNNTGNTNNANNANCNNNNANNNNNANNNNNNNNNCTNGNNCNTNNNNNCNNNTGGNGTGNNNNNNNNCNNNTNNNN | |||
NNNNNANNNNNNNNNNNNNNTANNNNNNTNANNNNNNNNNNNNNNNNNNNNNNNNNANNNNNNNTNTNNNNNNNNNNNNN | |||
NNNNNNNNNNGNNNNNAANNNNNTNNNCNNGTNAGTNNNNNNNNGGGNNNGGNNNNNNNNNCNNNNNCTGGNNNGNNNNN | |||
NNNNCNNNGNNNNNNNNNNNNNNNNNNNNNNNANCNNANNNNNNNNNNNNNNNNNN | |||
where N is an undefined amino acid. | |||
The BLAST software concluded that the highest match was to the Uncultured Oxalobacteraceae bacterium clone BGEL-OTU23 16S ribosomal RNA gene, partial sequence. | |||
Conclusions: | |||
Uncultured Oxalobacteraceae bacterium clone did not yield any direct results. But when just Oxalobacteraceae is researched a clear match is found. Oxalobacteraceae is a gram negative bacteria in the betaproteobacteria family (labome.org). In terms of morphology, Oxalobacteraceae is identical to the bacteria grown on the agar plates. It is dark purple, raised,wrinkled, umbonate, and with an undulated edge. | |||
References: | |||
QS and CRL Description. (n.d.). Retrieved March 2, 2015, from https://clims3.genewiz.com/Customer/OrderManagement/OrderResult.aspx?oId=4p6LZ4Isjwk= | |||
Basic Local Alignment Search Tool. (n.d.). Retrieved March 2, 2015, from http://blast.ncbi.nlm.nih.gov/Blast.cgi | |||
Oxalobacteraceae. (n.d.). Retrieved March 2, 2015, from http://www.labome.org/topics/organisms/bacteria/proteobacteria/betaproteobacteria/oxalobacteraceae-2524.html | |||
'''BW''' | |||
'''Vertebrates and Niches | |||
February 18th,2015''' | |||
Purpose: Identifying vertebrates and their place in the transect. | |||
Introduction: Five vertebrates were identified at the transect and then researched further for classification. | |||
Materials and Methods: Observation was used to find animals. | |||
Data and Observation: | |||
A squirrel ( phylum chordata, class mammalia, order rodentia, family sciuridae), a rat trap therefor a presumed rat (phylum chordata, class mammalia, order rodentia, family muridae), a bird specifically the American Tree Sparrow (phylum chordata, class aves, order passeriformes, family emberizidae), a hawk mentioned in podcasts of the walking tour of the AU campus (phylum chordata, class aves, order falconiformes, family accipitridae) and a presumed racoon as they are common on the AU campus (phylum chordata, class mammalia, order carnivora, family procyonidae) were found in the transect. Biotic aspects like trees and bushes with berries benefit the squirrel and the bird.The hawk would be benefited by biotic aspects like the rat and the bird would benefit from the found invertebrates. theAbiotic aspects, like a near by garbage can would benefit the raccoon and the rat. | |||
Conclusions and Further observations: | |||
Trophic levels, position in the food chain, can be seen in figure 1. | |||
Figure 1 is a food web of all of the organisms discovered in the transect: | |||
[[Image:Screen_Shot_2015-02-18_at_3.42.55_PM.png]] | |||
The hawk has the highest trophic level and is at the top of the food chain because it is a predator that can feed off of the rats and squirrels in the transect. Below the hawk is the raccoon and the rat who feed off waste and the squirrel and the sparrow who feed off invertebrates and plants. Together, the organisms make a community, two or more different species occupying the same area. Becasue of the central location and the large amount of plantae in the transect, carrying capacity is high. | |||
'''BW''' | |||
'''Invertebrates | |||
February 17th, 2015''' | |||
Purpose: Identifying invertebrates found in our transect and identifying them through characteristics and a dichotomous key in order to build the transect as a whole. | |||
Introduction: | |||
In the last lab, we took leaf and soil matter from the floor of out transect and placed it in a Berlese Funnel to collect invertebrates.Invertebrates are included in the kingdom Animalia and are further divided into nine phylas. Most of what is found in soil falls under the Arthropods phyla, insects and larvae. Arthropods can then be classified in five classes: arachnida, diplopoda, chilopoda, insect, and crustacea. Worms are also found in soil. Worms can also be found in soil and based off of their internal structure are classified as acoelomates (the digestive system does not have a coelom), pseudocoelomates (the body cavity in not totally lined), or coelomates (a completely lined coelom). | |||
Materials and Methods: | |||
The Berlese Funnel was taken apart and from the 50/50 ethanol/water solution 10-15 mLs of the top of the solution was placed into one dish and the remaining was placed into another dish. Both dishes were placed under a dissecting microscope. | |||
A dichotomous key was used to identify the organisms in the dish. | |||
Under the same, previously mentioned, dissecting microscope three types of worms were identified. A planaria sample, a nematode sample, and an earthworm sample were used. | |||
Data and Observation: | |||
The planaria cross section displayed a symmetrical body structure. The cross section lead to the assumption that the body contracts in order to move. | |||
The nematodes move sporadically without direction and have a very simple internal structure. Their movement could give rise to the idea that a simple body structure has no indication of location of food. | |||
The earthworm has a full coelomate structure. It's movements are controlled in independent by section. Upon dissection, it was found that organs are not fixed in placed allowing more flexibility. | |||
Five invertebrates were found. Their information is displayed in table 1. | |||
[[Image:Table_one_about_bugs.jpg]] | |||
Sample one can be seen in figure 1. | |||
Figure one: [[Image: Image:Flies and ticks.jpg]] | |||
The size and segmented body of sample 4 can be seen in figure two. | |||
Figure two: | |||
[[Image:Image:Rollie.jpg]] | |||
Conclusions and Further Directions: | |||
Now that we have on the topic of invertebrates, we can discuss vertebrates in the context of our transect and in specific subjects. Continuing to build our transect, we will discover animals and discuss the transect as their successful habitat. | |||
'''BW''' | |||
'''Plantae and Fungi | |||
February 11th,2015''' | |||
Purpose: Examining and classifying the plantae and fungi of a specific transect through vascularization, observation, and mechanism of reproduction. | |||
Introduction: From our transect, tall bushes, we took five samples. Figure one shows the samples before any microscopic observation. Clssification is based on four categories: vascularization, specialized structures,description, and mechanisms of reproduction.Further the category vascular system is based on that of a moss's rhizoids compared to an angiosperm's phloem and xylem components. Specialized structures include cuticles, the waxy layer on some leaves and any presence of their classification as an angiosperm, or flowering plant. Mechanisms of reproduction means their classification as a monocot or dicot if the cotyledon, or part of seed the delivers nutrients to the embryo prior to photosynthesis, forms as a single or paired structure. The samples vary between angiosperms, trees, and bushes. | |||
[[Image:Sample_total.jpg]] | |||
Materials and Methods: | |||
A sample of dead leaves and plant matter was placed in one bag and the five samples were placed in another bag and taken to the lab for further inspection. After basic observation, the samples were spliced in order to examine their vascular system under a microscope. A ruler was used to measure the original size of the sample. | |||
The dead plant matter was placed in a Berlese Funnel with a screen in order to collect invertebrates. Matter was placed in a funnel that was then taped to a 50 mL tube filled with 25 mL of 50/50 ethanol/water solution. The funnel, tube system was placed under a light and will be observed again in the next lab. | |||
Fungi was also observed and classified. Because our agar plates from lab three did not have any apparent fungi, we observed lab samples. | |||
PCR from last lab was placed in a agarose gel and then on a UV light box in order to confirm PCR for further identification. | |||
Data and Observations: | |||
Sample 1 is from a tree directly behind the rock with the plaque, naming the area. On the tree was a sign that identified it as a Japanese Snow Bell. Sample 2 was from one of the many small, circular bushes. This particular bush was directly next to a smaller rock in front of the rock with the plaque. Sample 3 is from one of the many trees with projection like branches that line the back of the transect.Sample 4 is from a ground plant under sample 3. And sample 5 is from the big tree in the center of the transect. All of the data observed can be found in table one. | |||
Table 1: [[Image:Table_i.jpg]] | |||
Figure 2 is an image of the vascular system of sample 2. | |||
Figure 2: [[Image:Sample2,_celery.jpg]] | |||
The observed bundles in a ring are clear. | |||
Figure 3 displays the vascular system of sample 3, the tree with projection like branches. | |||
Figure 3: [[Image:Sample_3_buggy.jpg]] | |||
Figure 4 displays the clear shape and scattered bundles of sample 4. | |||
Figure 4:[[Image:Sample_4_splits.jpg]] | |||
Figure 5 shows a split version of sample 5 in order to examine its vascular system. Compared to the other samples, we can see the strength of the branch. We also know that while this is only half of the branch, we know that it holds vascular bundles in a ring. | |||
Figure 5:[[Image:Sample_5_branch.jpg]] | |||
Later in lab, we observed fungi. Fungi can grow in structures called hyphae which then grow in structures called sporangia. Sporangia is important because it holds the fungi's reproductive struture, the spore. Under a disecting microscope, we classified three fungi as bread mold, rhizopus, and stotonifer zygospore. Figure 6 is an image of the classifies bread mold. | |||
Figure 6:[[Image:Fungi_microscope_.jpg]] | |||
We classified this sample as a fungus because of it's matte coloring and the longer particles that we assume are sporangias. | |||
Conclusions and Further Discussions: | |||
PCR will be processed so that it can be identified. Our PCR, shown in figure 7, displays a working PCR model in the second and fourth well. | |||
Figure 7: [[Image:Pcr_2_and_4.jpg]] | |||
Now that we have inspected and identified bacteria, plantae, and archea we can now inspect invertebrates. What we have learned of the transect so far will help us to identify invertebrates as we can build an ecosystem. The Berlese Funnel forces invertebrates to go downward away from the light and eventually fall into the ethanol solution which will hold them for identification is the next lab. | |||
'''BW''' | |||
'''January 29th, 2015 | |||
Identifying Bacteria''' | |||
Purpose: | |||
Identifying bacteria that was previously grown on Agar plates. | |||
Introduction: | |||
In the previous lab, Identifying Algae and Protists, we diluted samples from our Hay Infusion and placed them on agar plates with and without the anti-biotic Tetracycline in order to observe and identify bacteria from our transect. We predict identification of prokaryotes of the Domain Bacteria but also bacteria from the Domain Archea that are able to survive in the most extreme climates. While other bacteria may be killed by the Tetracylcine, the archea could survive. We are also expectant the some Domain Bacteria will also be resistant to the Tetracycline and survive on the plates containing anti-biotic. | |||
As we observe our Hay Infusion again, we can see that matter is settling. The potent smell of stagnant pond water is lessened, it isn't as green as it was last week, water has evaporated, and the berry and leaf have sunk to the bottom of the jar and are breaking down. Because the ecosystem of the hay infusion in itself is changing, it's appearance will change too. Slowly with the addition of water and milk powder from the original infusion, components are decomposing. As we continue to observe our hay infusion, it will continue on the same trend. | |||
Materials and Methods: | |||
Four nutrient agar plates, containing different dilutions from Lab II, with Tetracylcine and four nutrient agar plates without Tetracycline were observed. Firstly, the number of colonies on every plate was counted. They two types of plates were then observed for differences. Then, four samples, three from the plates without anti-biotic and one from a plate containing anti-biotic, were made into wet mounts and samples for gram staining. | |||
To make a wet mount a small sample is placed on a slide along with a drip of water and covered with a cover slip. | |||
A gram stain sample is made by taking a small sample from a colony, placing is on a blank slide with a drop of water, and passing each slide through a flame in order to dry the sample. Each sample is then drenched in crystal violet for one minute, iodine for one minute, 95% alcohol for 10-20 seconds, and safranin stain for 20-30 seconds. After each step the slide is rinsed in water. A kim wipe is then used to dry the slides. A 100x microscope and oil immersion is used to observe gram stains. | |||
For next weeks lab, we conducted the beginning of PCR. A colony of bacteria was placed in 100 microleters of water in a sterile tube, incubated for 10 minutes in a heat bath, then centrifuged for five minutes, the centrifuged sample was then added to a PCR tube with primer. The complete mixture was placed into the PCR machine. | |||
Data and Observations: | |||
Upon basic observation of the eight plates, we found thousands of colonies per millileter, figure one, that were purple, white, and orange, ranging in shape, size, and height. | |||
Figure One:[[Image:Colonies_counted.jpg]] | |||
Plates without anti-biotic had more colonies in more colors, plates with anti-biotic not only had much less colonies but they were only orange. | |||
Upon closer observation we found small, multi-cellular colonies true to their color. Figure two describes each colony and cell. | |||
Figure two: [[Image:Colonie_description.jpg]] | |||
The following images are taken of the exact slides at a 100x view. Figure three shows the staphylococci character and the purple color of colony one. | |||
Figure three:[[Image:Micro1.jpg ]] | |||
Figure four shows the milky color and part of the undulated edge. | |||
Figure four: [[Image:Micro2.jpg ]] | |||
Figure five shows smaller size of the cells in a staphylococci colony. | |||
Figure five:[[Image:Micro3.jpg]] | |||
Figure six is a colony that appears orange to the normal eye but transparent under the microscope. The cells are much bigger and are coccus in colonies. | |||
Figure six: | |||
[[Image:Micro4.jpg ]] | |||
We then looked at each gram stain to determine if it was positive or negative. | |||
Figure seven is the absolute positive gram stain of colony one. It's purple color tells use that the cells in colony one have a thick layer of peptidoglycan in their cell walls. | |||
Figure seven:[[Image:Micrograin1.jpg]] | |||
Figure eight clearly depicts the half purple half pink gram stain of colony two. | |||
Figure eight: [[Image:Micrograin2.jpg ]] | |||
In figure nine, we can see the small pink cells in the purple mass. Because the individual cells are pink, we can determine that the gram stain is negative meaning there is only a thin layer of peptidogylcan between two membranes. | |||
Figure nine: [[Image:Micrograin3.jpg ]] | |||
Figure ten also shows individual pink cells in a purple mass so we can also determine colony four as negative gram stain. | |||
Figure ten: [[Image:Micrograin4.jpg]] | |||
Conclusions and Further Directions: | |||
We can determine, through basic observation, that the Tetracycline kills all of the bacteria expect the orange. Because the orange colonies have to complete on the agar plates without Tetracycline, they are smaller. But on the Tetracycline plates, they do not need to complete for food, light with any other bacteria so they can grow larger. | |||
Tetracycline kills some bacteria by binding to the 30s ribosomal subunit. Without Tetracycline, t-RNA would bind to the 30s, A site of the ribosome thus promoting protein synthesis. The Tetracycline prohibits protein synthesis form occurring therefor killing the cell (Connell). Tetracyclines can kill many bacterias including e. coli, some forms of the flu, and some forms of Tuberculosis (Chopra & Roberts). | |||
But to determine what Tetracycline killed on we conducting the beginning stages of PCR in order to amplify DNA for identification. Later, we can identify our specific bacteria by gel electrophoresis. | |||
References: | |||
Connel, S. R. et al. (2003). Ribosomal protection proteins and their mechanism of tetracycline resistance. ''Antimicrobial Agents and Chemotherapy''47,12. | |||
Chopra, I & Roberts, M. (2002) Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance.''US National Library of Medicine.''''65''(2). | |||
'''January 28th, 2015 | '''January 28th, 2015 | ||
Identifying Algae and Protists''' | Identifying Algae and Protists''' | ||
Latest revision as of 15:43, 18 March 2015
Zebrafish Development in Retinoic Acid March 17, 2015
Purpose: Comparing zebrafish, in embryonic stages and beyond, under conditions containing retinoic acid and conditions without reintoic acid.
Introduction: Retinoic Acid is a metabolite of Vitamin A that is absolutely crucial to the normal development of chordate, high functioning, mammals. Retinoic acid functions as a stabilizer for Vitamin A in growth and development (Holland). Without enough retinoic acid or too much, mutations occur. In humans, too much retinoic acid can block the retinoic acid receptor resulting in predisposal to myeloid and lymphoid leukemia (Parrado et al.). In Zebra fish, an excess of retinoic acid can lead to mutations including a short or crooked tail, delayed hatching, ill formed or unformed valves on the heart and brain, and the absence of one or both eyes (Herrman). There are a number of reasons why zebra fish are used to study development. Their embryos are clear and development in the embryo is rapid compared to other species. Experimental variables can be injected directly into the water as zebra fish, in early developmental stages, accept their aquatic environment directly into their bloodstream (Institute of Molecular and Cell Biology).
Materials and Methods: 20 zebrafish eggs were placed in two separate dishes. In one dish, the control group, the zebrafish were placed in 10 Ml of water. In the other dish, the zebrafish were placed in 10 Ml of water and 5 Ml of retinoic acid. Every few days, the fish are fed a small amount of paramecium. Each week, the fish will be observed in order to measure size of fish, physical appearance of fish, and movement of fish all compared to the other group. On day seven of observation, three fish from each group was placed in preservative to keep them fixed in that stage of development.
Results: Day One (2/20): Four days after their initial placement, there was no hatching in either of the dishes filled with embryos. Though three of the embryos were dead in the acid, as indicated by the collection of a mold looking substance and the embryo's grey color. One embryo was dead in the control dish of just water. Day Two (2/23): Three more fish in the acid had died equalling a total of 16 fish left in the acid group. One more had died in the control equalling 18 total fish in the control group. Most of the fish had hatched. Day Three (2/26): Three more dead in acid. One more dead in control. All fish are very active. Retinoic is faster movement but is more delayed to start moving. Hatched fish in acid are more jittery in movement compared to the gliding movement of the control. Day Four (3/6): On the final day of observation, all were dead in acid. Nine had reached full form in comparison to the control group. Their pigment was black and dark blue with a cloudy complexion.There was no motility. Six more are dead in the control, nine fish are alive with normal movement. They are 5 cm in length, larger than the fish in retinoic acid.
Conclusions: Conclusions are drawn mostly from the day seven, fixed fish. Control fish are more structured, there is clear development organ development in a structured pattern. On 4x on the microscope fish are 15.5-17 mm. The back fin has very little development but structure is starting to form and there is no front fin development. The eye is 5x5.5 and there the pupil is transparent. The fixed fish on day 7 of the retinoic acid is 14 mm on 4x and has a grey pigment. Both back and side fins are starting to develop. Eye is 5x5.5 with no pupil.
The embryos hatched normally in both groups, in a matter of 5-6 days though the ratio of fish hatched in the water without retinoic acid is higher. Upon basic observation of the fish there is no major difference between the two groups. The retinoic acid has proven it's tested effects in the case of zebrafish embryos. Though,the retinoic acid has proven it's tested effects in the case of zebrafish embryos. Organ development is disrupted in fish exposed to retinoic acid. There is a solid color on the zebrafish in the control group while the color on the retinoic acid fish is less blotchy. The heart and brain are not in the same place in the retinoic acid fish as in the control group meaning that there are unformed or malformed valves of the heart and brain. Eyes are also a little larger in the fish exposed to retinoic acid. There is also marginal higher death rate in the retinoic acid fish. Too much exposure to retinoic acid does result serious birth defects.
References: Herrman, K. (1994). Teratogenic effects of retinoic acid and related substances on the early development of the zebra fish as assessed by a novel scoring system. Pergamon. 9.(3). 267-283.
Holland, L. Z. (2007). "Developmental biology: A chordate with a difference". Nature 447 (7141): 153–155.
Parrado, A., C. Chomienne, and R. A. Padua. (2000). Retinoic acid receptor alpha (RAR alpha) mutations in human leukemia. Leuk. Lymphoma 39:271-282. Institute of Molecular and Cell Biology. (2012). Zebrafish facility. A*Star Singapore.
BW
Identifying Bacteria March 2nd, 2105
Purpose: Identifying bacteria
Introduction: In previous labs we grew bacteria on agar plates with an without Tetracycline. After selecting a large, interesting sample the bacteria underwent PCR and was then placed on a gel electrophoresis. The PCR was then sent to an outside lab for sequencing specifically of the 16s gene.
Materials and Methods: The website genewiz.com was used to seuence the 16S PCR product from the bacteria specific to each group.Then the BLAST software was used to identify and classify each sequence to a bacteria.
Results: The original gel electrophoresis, shown in figure one, displayed the most successful PCR reaction in row two which was the sample chosen for sequencing.
After sequecning the rat data of the 16S PCR was:
NNNNNNNNNGNNTNNAATGCANNCGANANGGAGCANNANTTGTCTGGGGGAGNGCGACGGGNGAGTNTATATCGGAACGT ACCCTANGTGGGGGATATCGTCTCGAAAGTTACGCTAATACCGCATACGATCTAAGGTGAAAGCGGGGGATCGCAAGACC TCGCGCTCGTGGAGCGGCCGATATCTGATTATATAGTTGGTANGATAAAAGCCTACCAAGGCGTCGATCTGTAGCTGGTC TGAGAGGACGACCAGCCACACTGGGACTGAGACACGGNCNAGACTCCTACGGGAGGCAGCAGTGGGGAATTTTGNACAAT GGGCGAAAGCCTGATCCANCAATGCCGCGTGNNNNNAGAAGGCCTTCGGGTTGTAAANCTCTTTTNTNNGGCNANGANAA GGNNGANAGCTAATANCCCNNTGGTTNTTGACGNTACCTNNAGAATAAGCACCGGCTNNNNTACTNTGNNANCAGCCGCG GATAATACNNNAGGNGTGCTNANNNGNNTNNTNNGGNNATNNANNTGNNGNNGTNANNNNNNNNGCCGCNNNGNNNGGGG TNTNNNTGNTNNANNANCNNNNANNNNNANNNNNNNNNCTNGNNCNTNNNNNCNNNTGGNGTGNNNNNNNNCNNNTNNNN NNNNNANNNNNNNNNNNNNNTANNNNNNTNANNNNNNNNNNNNNNNNNNNNNNNNNANNNNNNNTNTNNNNNNNNNNNNN NNNNNNNNNNGNNNNNAANNNNNTNNNCNNGTNAGTNNNNNNNNGGGNNNGGNNNNNNNNNCNNNNNCTGGNNNGNNNNN NNNNCNNNGNNNNNNNNNNNNNNNNNNNNNNNANCNNANNNNNNNNNNNNNNNNNN
where N is an undefined amino acid.
The BLAST software concluded that the highest match was to the Uncultured Oxalobacteraceae bacterium clone BGEL-OTU23 16S ribosomal RNA gene, partial sequence.
Conclusions:
Uncultured Oxalobacteraceae bacterium clone did not yield any direct results. But when just Oxalobacteraceae is researched a clear match is found. Oxalobacteraceae is a gram negative bacteria in the betaproteobacteria family (labome.org). In terms of morphology, Oxalobacteraceae is identical to the bacteria grown on the agar plates. It is dark purple, raised,wrinkled, umbonate, and with an undulated edge.
References: QS and CRL Description. (n.d.). Retrieved March 2, 2015, from https://clims3.genewiz.com/Customer/OrderManagement/OrderResult.aspx?oId=4p6LZ4Isjwk=
Basic Local Alignment Search Tool. (n.d.). Retrieved March 2, 2015, from http://blast.ncbi.nlm.nih.gov/Blast.cgi
Oxalobacteraceae. (n.d.). Retrieved March 2, 2015, from http://www.labome.org/topics/organisms/bacteria/proteobacteria/betaproteobacteria/oxalobacteraceae-2524.html
BW
Vertebrates and Niches February 18th,2015
Purpose: Identifying vertebrates and their place in the transect.
Introduction: Five vertebrates were identified at the transect and then researched further for classification.
Materials and Methods: Observation was used to find animals.
Data and Observation: A squirrel ( phylum chordata, class mammalia, order rodentia, family sciuridae), a rat trap therefor a presumed rat (phylum chordata, class mammalia, order rodentia, family muridae), a bird specifically the American Tree Sparrow (phylum chordata, class aves, order passeriformes, family emberizidae), a hawk mentioned in podcasts of the walking tour of the AU campus (phylum chordata, class aves, order falconiformes, family accipitridae) and a presumed racoon as they are common on the AU campus (phylum chordata, class mammalia, order carnivora, family procyonidae) were found in the transect. Biotic aspects like trees and bushes with berries benefit the squirrel and the bird.The hawk would be benefited by biotic aspects like the rat and the bird would benefit from the found invertebrates. theAbiotic aspects, like a near by garbage can would benefit the raccoon and the rat.
Conclusions and Further observations: Trophic levels, position in the food chain, can be seen in figure 1.
Figure 1 is a food web of all of the organisms discovered in the transect:

The hawk has the highest trophic level and is at the top of the food chain because it is a predator that can feed off of the rats and squirrels in the transect. Below the hawk is the raccoon and the rat who feed off waste and the squirrel and the sparrow who feed off invertebrates and plants. Together, the organisms make a community, two or more different species occupying the same area. Becasue of the central location and the large amount of plantae in the transect, carrying capacity is high.
BW
Invertebrates February 17th, 2015
Purpose: Identifying invertebrates found in our transect and identifying them through characteristics and a dichotomous key in order to build the transect as a whole.
Introduction: In the last lab, we took leaf and soil matter from the floor of out transect and placed it in a Berlese Funnel to collect invertebrates.Invertebrates are included in the kingdom Animalia and are further divided into nine phylas. Most of what is found in soil falls under the Arthropods phyla, insects and larvae. Arthropods can then be classified in five classes: arachnida, diplopoda, chilopoda, insect, and crustacea. Worms are also found in soil. Worms can also be found in soil and based off of their internal structure are classified as acoelomates (the digestive system does not have a coelom), pseudocoelomates (the body cavity in not totally lined), or coelomates (a completely lined coelom).
Materials and Methods: The Berlese Funnel was taken apart and from the 50/50 ethanol/water solution 10-15 mLs of the top of the solution was placed into one dish and the remaining was placed into another dish. Both dishes were placed under a dissecting microscope. A dichotomous key was used to identify the organisms in the dish. Under the same, previously mentioned, dissecting microscope three types of worms were identified. A planaria sample, a nematode sample, and an earthworm sample were used.
Data and Observation: The planaria cross section displayed a symmetrical body structure. The cross section lead to the assumption that the body contracts in order to move. The nematodes move sporadically without direction and have a very simple internal structure. Their movement could give rise to the idea that a simple body structure has no indication of location of food. The earthworm has a full coelomate structure. It's movements are controlled in independent by section. Upon dissection, it was found that organs are not fixed in placed allowing more flexibility.
Five invertebrates were found. Their information is displayed in table 1.
Sample one can be seen in figure 1.
Figure one: File:Image:Flies and ticks.jpg
The size and segmented body of sample 4 can be seen in figure two.
Figure two: File:Image:Rollie.jpg
Conclusions and Further Directions:
Now that we have on the topic of invertebrates, we can discuss vertebrates in the context of our transect and in specific subjects. Continuing to build our transect, we will discover animals and discuss the transect as their successful habitat.
BW Plantae and Fungi February 11th,2015
Purpose: Examining and classifying the plantae and fungi of a specific transect through vascularization, observation, and mechanism of reproduction.
Introduction: From our transect, tall bushes, we took five samples. Figure one shows the samples before any microscopic observation. Clssification is based on four categories: vascularization, specialized structures,description, and mechanisms of reproduction.Further the category vascular system is based on that of a moss's rhizoids compared to an angiosperm's phloem and xylem components. Specialized structures include cuticles, the waxy layer on some leaves and any presence of their classification as an angiosperm, or flowering plant. Mechanisms of reproduction means their classification as a monocot or dicot if the cotyledon, or part of seed the delivers nutrients to the embryo prior to photosynthesis, forms as a single or paired structure. The samples vary between angiosperms, trees, and bushes.
Materials and Methods: A sample of dead leaves and plant matter was placed in one bag and the five samples were placed in another bag and taken to the lab for further inspection. After basic observation, the samples were spliced in order to examine their vascular system under a microscope. A ruler was used to measure the original size of the sample. The dead plant matter was placed in a Berlese Funnel with a screen in order to collect invertebrates. Matter was placed in a funnel that was then taped to a 50 mL tube filled with 25 mL of 50/50 ethanol/water solution. The funnel, tube system was placed under a light and will be observed again in the next lab. Fungi was also observed and classified. Because our agar plates from lab three did not have any apparent fungi, we observed lab samples. PCR from last lab was placed in a agarose gel and then on a UV light box in order to confirm PCR for further identification.
Data and Observations: Sample 1 is from a tree directly behind the rock with the plaque, naming the area. On the tree was a sign that identified it as a Japanese Snow Bell. Sample 2 was from one of the many small, circular bushes. This particular bush was directly next to a smaller rock in front of the rock with the plaque. Sample 3 is from one of the many trees with projection like branches that line the back of the transect.Sample 4 is from a ground plant under sample 3. And sample 5 is from the big tree in the center of the transect. All of the data observed can be found in table one.
Figure 2 is an image of the vascular system of sample 2.
Figure 2:  The observed bundles in a ring are clear.
The observed bundles in a ring are clear.
Figure 3 displays the vascular system of sample 3, the tree with projection like branches.
Figure 4 displays the clear shape and scattered bundles of sample 4.
Figure 5 shows a split version of sample 5 in order to examine its vascular system. Compared to the other samples, we can see the strength of the branch. We also know that while this is only half of the branch, we know that it holds vascular bundles in a ring.
Later in lab, we observed fungi. Fungi can grow in structures called hyphae which then grow in structures called sporangia. Sporangia is important because it holds the fungi's reproductive struture, the spore. Under a disecting microscope, we classified three fungi as bread mold, rhizopus, and stotonifer zygospore. Figure 6 is an image of the classifies bread mold.
We classified this sample as a fungus because of it's matte coloring and the longer particles that we assume are sporangias.
Conclusions and Further Discussions: PCR will be processed so that it can be identified. Our PCR, shown in figure 7, displays a working PCR model in the second and fourth well.
Now that we have inspected and identified bacteria, plantae, and archea we can now inspect invertebrates. What we have learned of the transect so far will help us to identify invertebrates as we can build an ecosystem. The Berlese Funnel forces invertebrates to go downward away from the light and eventually fall into the ethanol solution which will hold them for identification is the next lab.
BW
January 29th, 2015
Identifying Bacteria
Purpose: Identifying bacteria that was previously grown on Agar plates.
Introduction: In the previous lab, Identifying Algae and Protists, we diluted samples from our Hay Infusion and placed them on agar plates with and without the anti-biotic Tetracycline in order to observe and identify bacteria from our transect. We predict identification of prokaryotes of the Domain Bacteria but also bacteria from the Domain Archea that are able to survive in the most extreme climates. While other bacteria may be killed by the Tetracylcine, the archea could survive. We are also expectant the some Domain Bacteria will also be resistant to the Tetracycline and survive on the plates containing anti-biotic. As we observe our Hay Infusion again, we can see that matter is settling. The potent smell of stagnant pond water is lessened, it isn't as green as it was last week, water has evaporated, and the berry and leaf have sunk to the bottom of the jar and are breaking down. Because the ecosystem of the hay infusion in itself is changing, it's appearance will change too. Slowly with the addition of water and milk powder from the original infusion, components are decomposing. As we continue to observe our hay infusion, it will continue on the same trend.
Materials and Methods: Four nutrient agar plates, containing different dilutions from Lab II, with Tetracylcine and four nutrient agar plates without Tetracycline were observed. Firstly, the number of colonies on every plate was counted. They two types of plates were then observed for differences. Then, four samples, three from the plates without anti-biotic and one from a plate containing anti-biotic, were made into wet mounts and samples for gram staining. To make a wet mount a small sample is placed on a slide along with a drip of water and covered with a cover slip. A gram stain sample is made by taking a small sample from a colony, placing is on a blank slide with a drop of water, and passing each slide through a flame in order to dry the sample. Each sample is then drenched in crystal violet for one minute, iodine for one minute, 95% alcohol for 10-20 seconds, and safranin stain for 20-30 seconds. After each step the slide is rinsed in water. A kim wipe is then used to dry the slides. A 100x microscope and oil immersion is used to observe gram stains.
For next weeks lab, we conducted the beginning of PCR. A colony of bacteria was placed in 100 microleters of water in a sterile tube, incubated for 10 minutes in a heat bath, then centrifuged for five minutes, the centrifuged sample was then added to a PCR tube with primer. The complete mixture was placed into the PCR machine.
Data and Observations:
Upon basic observation of the eight plates, we found thousands of colonies per millileter, figure one, that were purple, white, and orange, ranging in shape, size, and height.
Figure One:
Plates without anti-biotic had more colonies in more colors, plates with anti-biotic not only had much less colonies but they were only orange.
Upon closer observation we found small, multi-cellular colonies true to their color. Figure two describes each colony and cell.
Figure two: 
The following images are taken of the exact slides at a 100x view. Figure three shows the staphylococci character and the purple color of colony one.
Figure three:
Figure four shows the milky color and part of the undulated edge.
Figure four: 
Figure five shows smaller size of the cells in a staphylococci colony.
Figure five:
Figure six is a colony that appears orange to the normal eye but transparent under the microscope. The cells are much bigger and are coccus in colonies.
Figure six:

We then looked at each gram stain to determine if it was positive or negative.
Figure seven is the absolute positive gram stain of colony one. It's purple color tells use that the cells in colony one have a thick layer of peptidoglycan in their cell walls.
Figure seven:
Figure eight clearly depicts the half purple half pink gram stain of colony two.
Figure eight: 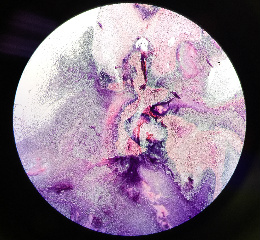
In figure nine, we can see the small pink cells in the purple mass. Because the individual cells are pink, we can determine that the gram stain is negative meaning there is only a thin layer of peptidogylcan between two membranes.
Figure nine: 
Figure ten also shows individual pink cells in a purple mass so we can also determine colony four as negative gram stain.
Figure ten: 
Conclusions and Further Directions: We can determine, through basic observation, that the Tetracycline kills all of the bacteria expect the orange. Because the orange colonies have to complete on the agar plates without Tetracycline, they are smaller. But on the Tetracycline plates, they do not need to complete for food, light with any other bacteria so they can grow larger. Tetracycline kills some bacteria by binding to the 30s ribosomal subunit. Without Tetracycline, t-RNA would bind to the 30s, A site of the ribosome thus promoting protein synthesis. The Tetracycline prohibits protein synthesis form occurring therefor killing the cell (Connell). Tetracyclines can kill many bacterias including e. coli, some forms of the flu, and some forms of Tuberculosis (Chopra & Roberts).
But to determine what Tetracycline killed on we conducting the beginning stages of PCR in order to amplify DNA for identification. Later, we can identify our specific bacteria by gel electrophoresis.
References: Connel, S. R. et al. (2003). Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrobial Agents and Chemotherapy47,12.
Chopra, I & Roberts, M. (2002) Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance.US National Library of Medicine.'65(2).
January 28th, 2015 Identifying Algae and Protists
Purpose: Examining and Identifying microorganisms in the Algae and Protist category.
Materials and Methods: The hay sample, form the precious lab, was used to make two wet mounts. One wet mount was from taken from the portion near the berry and the other was taken from the top to middle of the soil, water sample. The wet mounts were then examined under the microscope. Using a dichotomous key, a total of six microorganisms were identified.
Data and Observations:
Upon the opening of the hay sample, that had been brewing in the lab for a week, we noted the smell was something like stagnant water. As shown in figure one and two, the soil had settled to the sides and bottom of the jar.
Figure 1:
 Figure 2:
Figure 2:
 G_42
Unlike other samples, there was no present mold or green shoots. After basic observations, the two samples were taken and made into wet mounts. By taking a sample closer to and farther away from vegetation we can see if algae and protists differ based on their habitat. One micro-organism closer to vegetation, and therefor direct food source, may be larger than the same micro-organism away from vegetation.
From the wet mount of the sample from the top or middle of the infusion, the identified organisms were Burasaia Truncatella, Gonium, and Pelomyxa (Figure 3). All three were motile, though the Pelomyxa was much slower than the others. I could not tell if they were photosynthesizing. The Burasaia Truncatella was larger than the other two.
Figure 3:
G_42
Unlike other samples, there was no present mold or green shoots. After basic observations, the two samples were taken and made into wet mounts. By taking a sample closer to and farther away from vegetation we can see if algae and protists differ based on their habitat. One micro-organism closer to vegetation, and therefor direct food source, may be larger than the same micro-organism away from vegetation.
From the wet mount of the sample from the top or middle of the infusion, the identified organisms were Burasaia Truncatella, Gonium, and Pelomyxa (Figure 3). All three were motile, though the Pelomyxa was much slower than the others. I could not tell if they were photosynthesizing. The Burasaia Truncatella was larger than the other two.
Figure 3:
 At the berry, we identified Copidium Sp., Blepharism Sp, and Chlamydomanas (Figure 4). The Blepharism Sp. was interesting as it was long, non-motile, and bring pink completely unlike the other identified samples from the same wet mount. The Copidium Sp. seemed to dodge in and out of view under the protection of particles from the berry. Of the three, the Copidium Sp. was the smallest.
Figure 4:
At the berry, we identified Copidium Sp., Blepharism Sp, and Chlamydomanas (Figure 4). The Blepharism Sp. was interesting as it was long, non-motile, and bring pink completely unlike the other identified samples from the same wet mount. The Copidium Sp. seemed to dodge in and out of view under the protection of particles from the berry. Of the three, the Copidium Sp. was the smallest.
Figure 4:

Conclusions and Future Directions: From the original hey infusion, We prepared for next week's lab by making serial dilutions. In each tube, we placed 100 mLs of sterile broth. In the first tube, we measured 100 uLs of the hay infusion and placed 100 uLs on a plate with and without Tetracycline. From the first tube, we measured another 100 uLs and placed it in the second tube, thus diluting the solution. The process was repeated four times so that a total of eight plates were made. A diagram of serial dilution is shown below (Figure 5) File:Serial dillution .JPG
RW
January 24th, 2015 Tall Bushes at the Amphitheater
Purpose: Observing, testing, and inspecting a transect for abiotic and biotic components.
Materials and Methods: A 20 by 20 meter transect will be inspected throughout the assignment. The transect, "tall bushes" is a hilly area directly next to the American University amphitheater. Once at the transect, we took a 50 mL sample of the soil near a discovered rat trap, a berry, and a leaf. Figure 1 is an illustrated map of the 20 by 20 transect. The 50mL sample was then used in a Hay Infusion in which 10 to 12 grams of the soil sample was placed in a plastic jar and mixed with .1 gram of dried milk and 500 mL of sterilized water.
Figure 1:
Data and Observations: The transect is on a hill and is covered in foliage either fallen on the ground, in the round bushes, or in trees behind the bushes. Upon further examination, we discovered a rat trap and that the bushes were growing berries. A rock with a plaque marks the area. The following images are of the area.
Biotic (live) components include squirrels, bushes, trees, birds, and insects. The squirrels and birds moved around the transect in the bushes, trees, or on the floor.The trees are behind the bushes, or Northern in relation to them. Insects were found in accordance to their webs, under the bushes. Abiotic (non-living components) include rocks, a plaque, a rat trap, dirt, and snow. The rocks, or mainly one rock, is in the Southeastern corner of the transect and on it is a plaque. The rat trap was found a few feet behind the rock. As of the week of January 14th, snow bordered the transect.
Conclusions and Future Directions: We will continue to monitor the transect so that we can see it in other types of conditions. I predict that we will see more wildlife and more evidence of colder weather like fallen leaves and browning bushes as the assignment continues.
RW