User:Saroj Pandey/Notebook/SNP PCR optimization/2014/09/18
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |
gDNA extraction from buccal cavityTissue Preparation 1. Gargle with 14 ml 0.9% NaCl. Apply toothbrush to the inner lining of buccal cavity. Collect the tissue suspension in a tube. 2. Centrifuge at 5000 rpm for 20 minutes. 3. Discard supernatant and transfer pellet in a 1.5 ml eppendorf tube. Add 0.9% NaCl to make up the volume to 1.5 ml. 4. Centrifuge at 12000 rpm for 4 minutes. 5. Discard supernatant and use pellet for DNA extraction.
1. Add 250 µl buffer PB incl. 20 µl proteinase K. Vortex thoroughly at max. speed for 15 s. 2. Incubate the tube at 52°C for 45 min. Vortex occasionally. 3. Add 250 µl buffer AB and vortex for 5 s. Transfer solution to a spin column by pipetting. 4. Centrifuge loaded column at 13000 rpm for 30s. Discard flow-through. 5. Add 400 µl buffer WB to the spin column. Centrifuge at 13000 rpm for 30s. Discard flow-through. 6. Wash the spin column with 400 µl 70% ethanol by centrifugation at 13000 rpm for 3 min. Carefully remove the tube and discard flow-through. 7. Transfer the column to a 105 ml tube. Place 100 µl buffer EB preheated to 70°C in the centre of the column, close the lid and incubate for 1 min. Centrifuge at 13000 rpm for 1 min to elute DNA.
1. Taster: 58.3 ng/μL (260:280 - 1.77) 2. Non-taster: 25.7 ng/μL (260:280 - 1.74) PCR for gDNA amplificationProduct Size: 992 bp Pair Any: 6.0 Pair End: 2.0 TAS2R38_gaF: ATCCGTGATGCTGTGCTATG Length: 20 bp Tm: 59.7 °C GC: 50.0 % ANY: 4.0 SELF: 0.0 TAS2R38_gaR: GCATCCCAGAAGAAACCAGA Length: 20 bp Tm: 60.2 °C GC: 50.0 % ANY: 2.0 SELF: 0.0
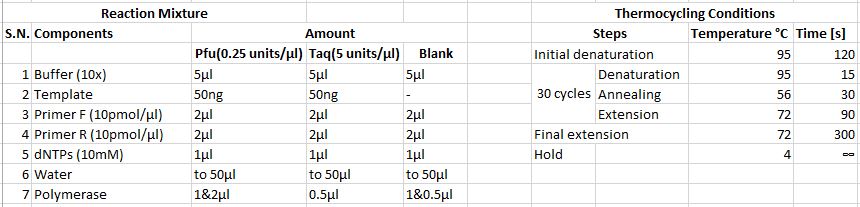
PCR 1

PCR 2
  Agarose gel electrophoresis
Sample mixtures were loaded to individual wells and the chamber was covered
Observation
DNA concentration 1. T3: 520.9 ng/μL (260:280=1.79) 2. N3: 406.7 ng/μL (260:280=1.83) - Samples T3 and N3 were sent for sequencing
| |