User:Student 66/Notebook/Biology 210 at AU
March 3, 2016
Zebrafish Experiment
Introduction The purpose of the following experiment was to observe the reaction of continuous movement and the physiological stress on the embryological development of zebrafish. The hypothesis was that the continuous, constant motion on the experimental group would lead to irregular growth, anxious behavior, and premature death due to physical stress.
Materials and Methods
I. Setting up a Control/Experimental Samples
Day 1: The experiment began setting up both the control and experimental samples.
The following process was completed by the end of the lab period.
• Two petri dishes were obtained. Two control (20 mLs of Deerpark water) and two treated/experimental (20 mLs of Deerpark water containing treatment) were set up and labeled.
• 20 healthy translucent embryos were obtained. One healthy embryo was placed into the small pivet of each petri dish with the help of a transfer pipett (When transferring embryos, it was important to be aware of minimizing the amount of water transferred with the animals).
• Next, an observation schedule was created using Google Sheets. This was used to record observations over the next two weeks.
• Finally, the experimental samples were placed onto the Hybrid Capture ™ System and the control samples were set aside. (A lable was made indicating that an experiment was being done and nobody should disturb the experiment).
• All other materials were disposed of correctly before lab was concluded.
II. Removing Empty Egg Cases/ Observation & Care for Days 4-5, 7 and 11.
Throughout the experiment, the fish needed to be fed, observed and often times preserved. Specific things needed to be completed on days 4-5, day 7 and day 11.
Making Measurements and observations: • Each day (not including weekends) the state and developmental stage of the zebrafish were evaluated with the use of dissecting scopes and depression slides.
o The following was counted for each dish on each observation day:
• Number of dead eggs
• Number of living embryos still in egg cases
• Number of living hatchlings (larvae)
• Number of dead hatchlings
• When a zebrafish was concluded to be dead, it was preserved by placing it in 0.02% tricaine solution. The TA then transferred them to a vial of 4% formaldehyde. Each vial was labeled separately for each sampled time point.
• All observations were recorded into the Google Sheet.
• Each day the zebrafish were fed approximately 15mL of specific zebrafish food.
• Any debris in the petri dishes were removed and replaced with more water.
Day 4-5:
• Empty egg cases were removed. (The empty cases will grow mold).
• Embyros/larvae from each plate were observed with the compound microscope and depression slide. A few drops of sample with the organisms were placed on a depression slide with the use of the 4X objective. The following was recorded:*
o Degree of body and tail pigmentation (melanophores)
o Eyes and eye movement
o Heart and possibly heart rate
o Pectoral fin development
o Yolk sac size (absorbed by day 5-7)
o Development of the swim bladder
o Development of the mouth (protruding jaw)
o General movement
- Repeat this process on Days 7 and 11.
• An additional 10 mLs of water and 25 mLs of fresh water were added to the petri dish pivets. (The volume was adjusted as necessary to be sure the control and experimental petri dishes were treated the same).
• Dead embryos were saved in paraformaldehyde.
• After this day, the number of dead larvae was continuously checked and recorded.
Day 7:
• 3 larvae was fixed up from the control and 3 from the test dish.
• A dropper was used to get them into a tube containing tricaine solution. The TA added the paraformaldehyde and stored the samples.
• Another fixation was completed on day 14 and measurements were made.
• On the final day, the following was observed:
o All the observations from day 4-5.
o Measurements of the length of the tail
o Measurements of the length of the entire larvae
o Measurements of the diameter of the eyes
Results
Table 1: Data Collected for Control Zebrafish
Table 2: Data Collected for Experimental Zebrafish
Both Tables 1 & 2: The tables above show the qualitative observations of the zebrafish from developmental to larval stage. Those characterized as "motile" were seen to be swimming actively on their own during observation. Those characterized as "nonmotile" were sedentary, but with agitation they moved around. Overall, the control fish on average survived until Day 14. The experimental fish did not survive past Day 11 during observation.
Conclusion
Zebrafish, from developmental to larval stage, are impacted by continuous movement. The constant shaking of the zebrafish led to physiological stress - which led to earlier death.
MR
Week 6: 16S Sequence Analysis
February 24th, 2016
Introduction The purpose of the following experiment was to use primers and PCR to amplify the 16S rRNA gene in one of the two nutrient agar plates with and without tetracycline. The hypothesis was that the gene sequence would be diverse and the sequence would be specific to the species found in the agar plates.
Materials and Methods
Procedure I: Setting up PCR for 16S Amplification
1. Label 2 PCR tubes with the specific transect number, colony identifier, and group number.
2. Record the labeling system in a lab notebook.
3. Add 25 micro liters of primer/water mixture to a labeled PCR tube. Mix the two to dissolve the PCR bead.
4. Next, use a sterile toothpick to up a small amount of a bacterial colony that you wish to characterize.
5. Completely submerge the toothpick in the primer/water mix of a PCR tube and twist it for approximately 5 seconds. Throw out the toothpick in the correct container.
6. Cap the tube and place it into the PCR machine.
7. Repeat this process using a fresh toothpick.
8. Next lab period, you will run the PCR products onto an agarose gel.
Procedure II: Running the PCR products onto an agarose gel
1. Retrieve PCR products.
2. Pipette approximately 5 ul of the PCR product into the agarose gel.
3. Place agarose gel underneath the specific light and observe results.
Procedure III: Analyzing the PCR products on the agarose gel
1. You will be provided the 16S Nucleotide Sequence.
2. Access the following website: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch
3. Copy and past the sequence into the specified box called "Enter accession number(s), gi(s), or FASTA sequence(s)". Make sure to start/end the sequence 30 letters in. Hit "Blast."
4. After receiving the results, observe and make notes of the "Distribution of 100 Blast Hits on the Query Sequence."
5. Scroll down to "Sequences producing significant alignments"
6. Select the first description and record results.
Reults
Nucleotide Sequence CTTGCTGCTTCGCTGACGAGTGGCGGAC GGGTGAGTAATGTCTGGGAAACTGCCTGATGGAGGGGGATAACTACTGGAAACGGTAGCTAATACCGCATAACGTCGCAA GACCAAAGAGGGGGACCTTCGGGCCTCTTGCCATCAGATGTGCCCAGATGGGATTAGCTAGTAGGTGGGGTAACGGCTCA CCTAGGCGACGATCCCTAGCTGGTCTGAGAGGATGACCAGCCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAG GCAGCAGTGGGGAATATTGCACAATGGGCGCAAGCCTGATGCAGCCATGCCGCGTGTATGAAGAAGGCCTTCGGGTTGTA AAGTACTTTCAGCGGGGAGGAAGGCGATGTGGTTAATAACCACGTCGATTGACGTTACCCGCAGAAGAAGCACCGGCTAA CTCCGTGCCAGCAGCCGCGGTAATACGGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCACGCAGGCGGTC TGTCAAGTCGGATGTGAAATCCCCGGGCTCAACCTGGGAACTGCATCCGAAACTGGCAGGCTTGAGTCTCGTAGAGGGGG GTAGAATTCCAGGTGTAGCGGTGAAATGCGTAGAGATCTGGAGGAATACCGGTGGCGAANGCGGCCCCCTGGACGAAGAC TGACGCTCANGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGTCGACTTGG AGGTTGTGCCCTTGAGGCGTGGCTTCCGGAGCTAACGCGTTAAGTCGACCGCCTGGGGAGTACGGCCGCAANGTTAAAAC TCNNTGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCNATGCAACGCGAAGAACCTTACCTGGTCT TGACATCCACAGAACTT
Figure 1: Result from running the PCR products on an agarose gel (Week 3)
Figure 2:Distribution of 100 Blast Hits on the Query Sequence
Conclusion
Our hypothesis was correct that the gene sequence would be diverse and specific to the species from transect #3 agar plates. We were able to identify the strain Salmonella bongori strain SL18 16S ribosomal RNA gene, partial sequence, from our gel sequence. Slmonella bongori is a pathogenic bacterium and "it is a Gram negative rod-shaped bacterium (bacillus) which causes a gastrointestinal disease called salmonellosis, characterized by cramping and diarrhea. It is typically considered a microbe of cold-blooded animals, unlike other members of the genus, and is most frequently associated with reptiles. [1]
References
[1] https://en.wikipedia.org/wiki/Salmonella_bongori
MR
Week 5: Invertebrates and Vertebrates February 11th, 2016
Introduction The purpose of this experiment was to examine our samples from the Burlese Funnel that we had set up a week prior and characterize the different kinds of invertebrates in Transect #3. It was hypothesized that there would be a variety of arthropods. Considering the changing temperatures over the past month, we assumed that there would not be many invertebrates present. However, we were only able to find four organisms and identify them with a dichotomous key. We did this by looking at the plates with the collected liquid underneath the dissecting microscope.
Materials and Methods Procedure: Analyzing the Invertebrates Collected with the Berlese Funnel
1. Retrieve the Berlese Funnel that was constructed a week prior to today.
2. Carefully pour the top 10-15 mL of 50% ethanol and organisms into a petri dish. Pour the remaining liquid into a second dish.
3. Examine the two under a dissecting microscope. Poke around with a probe to see the organisms clearer.
4. Identify the class of Arthropoda invertebrates (at least five) observed and record it into a table. Use a dichotomous key to help characterize which invertebrate it is. Also include a description of the salient features that helped identify the organism.
5. Measure the organisms length, how many of them there were, and name its phylum and class. Record this information into a table.
Results
Table 1: Invertebrates Found in Transect #3 & their Descriptions
Figure 1: Invertebrates in Transect #3 : Flies, Sucking lice, Termites, Springtail
Table 2: Vertebrates Found in Transect #3
Figure 2: Food Web of Transect #3
Conclusion
Transect #3 displayed a handful of organisms between both invertebrates and vertebrates all living together in one community. We learned that there were many microorganisms existing in this transect that we would never see with the naked eye, and they all play a role in the different trophic levels. Carry capacity is representative in this transect as there are predator-prey relationships that exist, as well as limited resources, that create a pattern of life within this transect.
MR
Week 4: Plantae and Fungi
February 4th, 2016
Introduction The purpose of this experiment was to identify and characterize plant samples from Transect #3. It was hypothesized that we would find a similar, but abundant amount of plants within our transect.
Material and Methods
Procedure I: Collect 5 plant samples from Transect #3
1. Collect three Ziploc bags and go to Transect #3
2. Collect a leaf litter sample (about 500g) and place it into one bag. This will be used later on for the set up of a Berlese funnel.
3. Collect samples of five plants (making sure to not damage the plant). Ensure that each plant is different from the other.
4. Take a photo of a tree within the transect. Also, collect old leaves or branches on the ground - this will be used to identify the genus.
5. Finally, collect a range of seeds, pine cones, and flowers that can be found in the transect.
Procedure II: Plant Vascularization
1. Observe the moss, Mnium, and record the heigh of the plant. Compare this height to the height of the lily plant stem.
2. Observe the cross section slide of the lily stem. Identify the xylema and phloem layers.
3. Retrieve plants collected in transect #3. Prepare each plant to be observed under the microscope.
4. Characterize the vascularization in each of the plants collected from transect #3. Record height and prepare an look at a cross section from each.
Procedure III: Presence of Specialized Structures
1. Describe the shape, size, and cluster arrangement of the leaves collected in transect #3. Record information in a table.
Procedure IV: Mechanisms of Plant Reproduction
1. Retrieve the seeds collected in transect #3. Dissect the seeds.
2. Identify the seeds as either monocot or dicot. Record results.
3. Make note if there is any evidence of flowers or spores.
Procedure IV: Setting up the Berlese Funnel to Collect Invertebrates
1. Begin by pouring 25 mL of the 50:50 ethanol/water solution into a 50 mL conical tube.
2. Fit a piece of screening material to the bottom of the funnel to prevent any leaf litter falling in. If need be, tape the sides of the screen.
3. Empty the bag of leaf litter into the top of the funnel.
4. Use a ring stand to hold the funnel and tube of ethanol.
5. Tape around the outside the base of the funnel and the tube so that the ethanol will not evaporate.
6. Place the funnel and ring stand underneath a 40 watt lamp (making sure the incandescent bulb is about 1-2 inches from the top of the leaf litter).
7. Make sure everything is covered with foil.
8. Leave the Berlese Funnel in that position until the next lab.
Results
Image 1: Five plants taken from Transect #3
Image 2: Location of Plants in Transect #3
Image 3: Tree in Transect #3
Table 1: Characteristics of Plants Collected from Transect #3
The seeds that we brought back from our transect were wheat seeds. Their vascularization was monocot. There was evidence of flowers because there were flowers on top of bud found on the bush.
Fungi sporangia are asexual sacs where spores are formed inside of. Fungi sporangia is important because it is a way that fungi reproduce. When examining the sample with the dissecting microscope, we decided that it was the fungi zygomycetes.
Conclusions
Our hypothesis was correct. There are is a wide variety of plants within Transect #3. The characteristics of each plant were different from one another but their mechanisms of reproduction were the same.
-MR
Week 3: Characterizing Colonies Using Gram Stains and Wet Mounts
Introduction The purpose of the following experiment was to identify and characterize the colonies and cells of bacterial species collected in Transect #3 after having left the serially diluted agar plates to grow over the course of a week. We were able to make our observations with the aid of a wet mount to clearly see the behavior of the cells under the microscope. Following that, we used the technique called Gram stain to observe the cell walls and determine the amount of peptidoglycan in the bacteria. Our hypothesis was that the the bacteria found on the regular agar plates [tet(-)] would obtain a wider variety and amount of bacteria than the bacteria observed on the agar plates with tetracycline [tet(+)].*
What we found was that our serial dilutions (from a range of 10^-3 to 10^-9 - odd numbers only) supported our hypothesis. The group without tetracycline displayed more colonies: 10^-3=880 10^-5=213 10^-7=150 and 10^-9=80. We can make the assumption that the greater the dilution number the more resistant it is to bacterial and fungal growth.
Our second group with tetracycline displayed less colonies: 10^-3=28 10^-5=1 10^-7=0 and 10^-9=0. We can make the assumption that the lower the tetracycline dilution, the higher the chance of forming colonies on agar plates.
When observing the Hay Infusion, we found that there were a couple changes in smell and appearance. The color of our Hay Infusion had changed from a dark shade of green to a light. There was also a lack of water compared to when we first observed our Hay Infusion. The smell was just as bad as before but less pungent - we believe that this is due to the breaking down of microorganisms by the bacteria.
* Tetracyclines are inexpensive antibiotics, which have been used extensively in the prophlylaxis and therapy of human and animal infections and also at subtherapeutic levels in animal feed as growth promoters. Tetracycline are broad-spectrum agents, exhibiting activity against a wide range of gram-positive and gram-negative bacteria, atypical organisms such as chlamydiae, mycoplasmas, and rickettsiae, and protozoan parasites.Tetracyclines inhibit bacterial protein synthesis by preventing the association of aminoacyl-tRNA with the bacterial ribosome.
Chopra,I. June 2001 "Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance". Journal of Microbiology and Molecular Biology Review. Volume 65(2).
Materials and Methods
1. Collect the 8 agar dishes that were set aside the week before. Observe colony morphology and take note of any changes.
2. Retrieve 2 samples from (+) and 2 samples from (-) and place them on glass slides.
3. Begin the Gram Stain process by evaporating excess water with the air of a Bunsen burner.
4. Continue the Gram stain process from the sheets posted on the lab tables. You will be using Crystal Violet, Iodine, Alcohol, and then Safranin Stain, and rinsing with deionized water in between steps.
5. Let the glass plate dry completely and proceed to observe under the microscope. Record whether or not are Gram positive or negative by basing it off the color of the peptidoglycan.
6. Prepare wet mounts for the chosen samples from the agar dishes and observe and record your observations of the behavior of bacterial species.
7. Take samples of bacteria, (-) and (+), and place it into two PCR tubes for genetic analysis.
Results
Table 1: Serial dilution results table
Table 2: Serial Diluted Agar Plates
Table 3: Gram stains: Plates E7, E9, E5, E3
Conclusion
Our hypothesis appeared to be correct. There was a more diverse and abundant amount of bacterial growth on the agar plates that did not obtain tetracycline [tet(-)].
-MR
What a great entry! Keep it up. -Pragati
Week 2: Hay Infusion
Introduction The purpose of the following experiment to observe the unicellular eukaryotic organisms growing on the top, middle, and bottom of Transect #3's Hay Infusion Culture. We used a dichotomous key to identify the protists and algae found in Transect #3. Our hypothesis would be that the different niches (top, middle, bottom) would have different protists and algae due to the different abiotic and biotic components in each layer.
Materials and Methods
1. Observe the Hay Infusion Culture. Make any notes on the appearance, smell, and color.
2. Identify the different layers of the Hay Infusion Culture and describe each.
3. Observe a sample from each niche.
4. Measure each organism and identify each using a dichotomous key.
5. Mix the Hay Infusion Culture jar by swirling it around with the lid on.
6. Collect and label four tubes of 10mLs sterile broth with 10^-2, 10^-4,10^-6,10^-8. Also collect a micropippetor and set is at 100micro Liters.
7. Collect four nutrient agar and four nutrient agar plus tetracycline plates. Label the plates with tetracycline with "tet" - always on the side of the plate.
8. Add 100 micro Liters from the culture to the 10mLs of broth in the tube labeled 10^-2. Swirl the inoculated tube throughly.
9. Repeat this process twice more to make the 10^-6 and 10^-8 dilutions.
10. Pipette 100 micro Liters from the 10^-2 tube onto the nutrient agar plate labeled 10^-3.
11. Repeat this process on the +tet plate labeled 10^-3.
12. Repeat this procedure with the 10^-4 dilution on the 10^-5 plates, 10^-6 dilution on the 10^-7 plates and the 10^-8 dilution on the 10^-9 plates.
13. Set the agar plates aside (side up) onto a rack. Leave them there at room temperature for a week.
Results
Observations of Hay Infusion Culture

Figure 1: Transect #3 Jar
Figure 2: Transect #3 Jar from above
1. The smell was pungent, like sewer. Almost a mold-like smell. Murky in appearance. The water was a light brown/green.
2. There was life on top of the liquid, as seen by the mold film on the surface of the water.
Niches of our jar:'
Figure 3: Jar Niches - Three distinct niches including: top, middle, bottom
4. Protists and algae present
On the top layer: Gonium, pandorina, colpidium, volvox
Middle layer: Volvox, paramecium bursaria, spirostomum, eugirna
Bottom layer: Blepharisma, didinium cyst, chilomonas sp,, spirostomum, pandorina colony
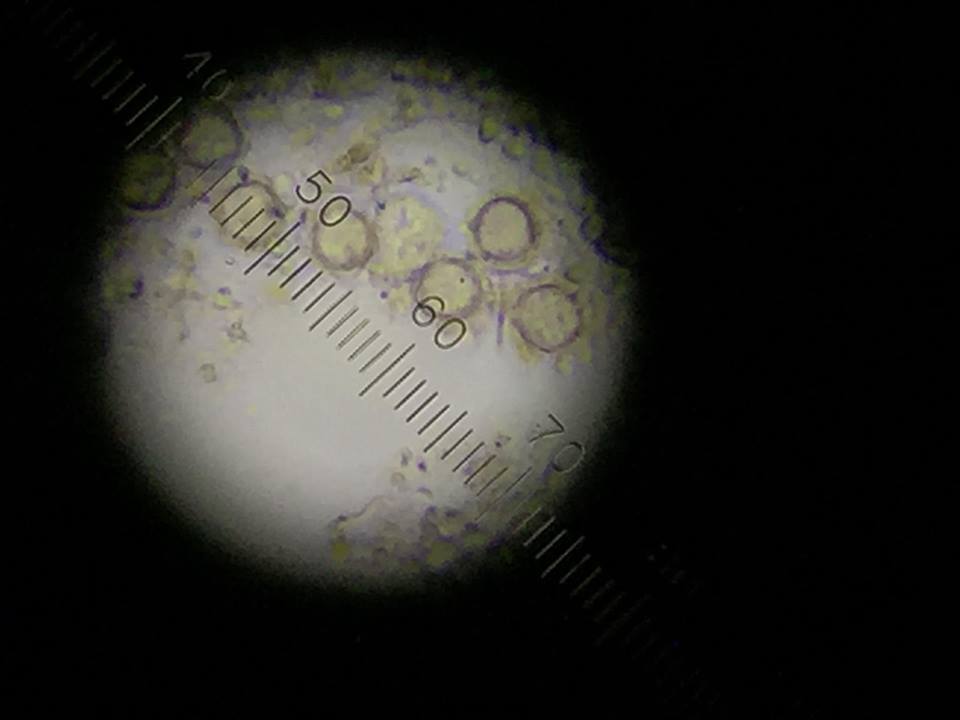 Figure 4: Gonium found top niche.
Figure 4: Gonium found top niche.
The following organism was found in the top layer of our jar. Observed was the gonium (colony up to 90 um).
 Figure 5: Volvox found middle niche.
Figure 5: Volvox found middle niche.
The following organism was found in the middle layer of our jar. Observed was the volvox (350-500 um).
 Figure 6: Didinium cyst found on bottom layer.
Figure 6: Didinium cyst found on bottom layer.
The following organism was found in the bottom layer of our jar. Observed was the didinium cyst.
6. None of the following above were motile.
7. If the Hay Infusion Culture "grew" for another two months, it is likely that the protozoans would consume the algal organisms. It is likely that mold would also begin to grow.
Serial Dilution:
Figure 7: Serial Dilution Process
Conclusion
Our hypothesis appeared to be correct. There were different protists and algae found in the top, middle, and bottom niches. This provides insight that certain organisms thrive in different niches of abiotic and biotic features.
-MR
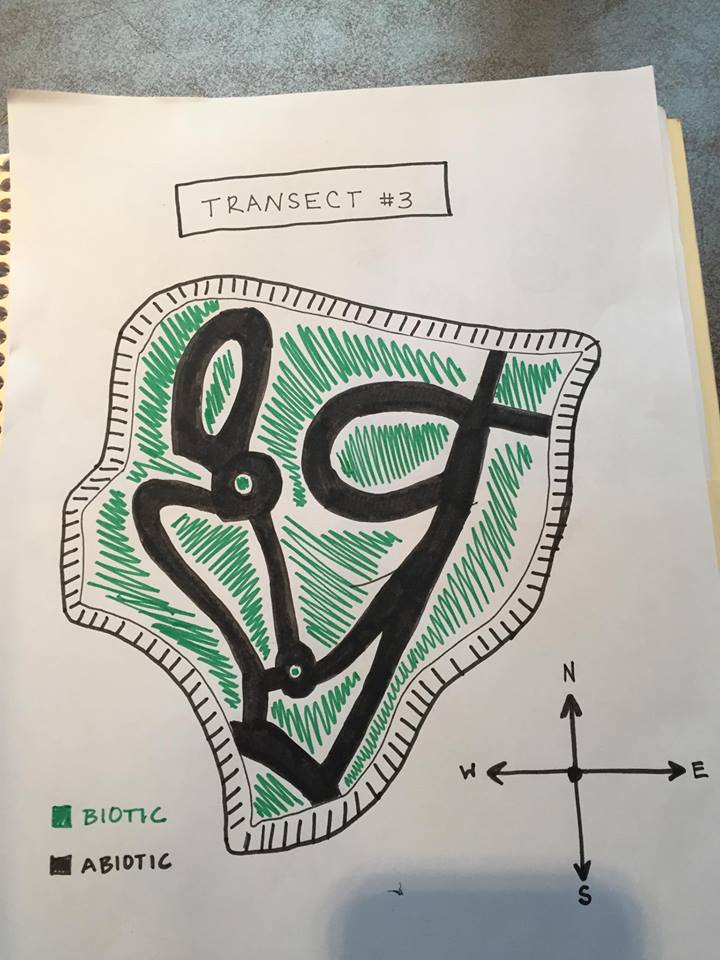
Transect #3
Week 1: Transect Observation
Location:
Aerial view of Transect showing Abiotic and Biotic areas:
Transect #3 sits between Bender Arena and Hughes Residence Hall. The transect lies just above the amphitheater and before the road. The area consists of a few benches, light poles, garbage can, and a concrete pathways winding about the garden. The ground is relatively flat only to be interrupted with a staircase. The soil is dark with mulch and dead leaves on top of it. There are a variety of trees and plants assorted throughout the transect as well.
Abiotic features: 1. Metal benches 2. Mulch 3. Rocks 4. Dirt 5. Lamp poles
Biotic features: 1.Leafy plants 2. Leaves 3. Weeds 4. Tall trees 5. Small insects
Madeline_B._Rohrbacher