User:Suzanne Shaffer/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| Line 213: | Line 213: | ||
|- | |- | ||
| ||Protruding mouth|||||| | | ||Protruding mouth|||||| | ||
|- | |||
| | |||
|} | |||
After Data was collected for the 14 days, the fixed zebra fish were examined to compare the 0.3%NaCl and 0.03% NaCl to the control to see what in the development stages were affected. | |||
{| | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''26-Feb''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''Dead''' | |||
|- | |||
| ||0.03%-1||0.03%-2||0.3%-1||0.3%-2||Control-1||Control-2||0.3%-1-4 | |||
|- | |||
| Fish length (micrometers||2500||2500||2000||2000||2625||2625||1250 | |||
|- | |||
| Eye size (Micrometers||250||250||200||200||275||275||250 | |||
|- | |||
| | |||
|} | |||
{| | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''28-Feb''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
|- | |||
| ||Control-1||Control-2||Control-3||0.3%-1||0.3%-2||0.3%-3 | |||
|- | |||
| Fish length (micrometers||2625||2625||2625||1250||1250||1250 | |||
|- | |||
| Eye size (Micrometers||375||375||375||225||225||225 | |||
|- | |||
| | |||
|} | |||
{| | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
| align="center" style="background:#f0f0f0;"|'''4-Mar''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
|- | |||
| ||0.03%||Control | |||
|- | |||
| Fish length (micrometers||2625||2500 | |||
|- | |||
| Eye size (Micrometers||225||250 | |||
|- | |- | ||
| | | | ||
Latest revision as of 08:55, 22 March 2014
2/27/2014
Question/Objective:
To understand the different stages of development in different organisms. To understand how Zebra Fish embryonic development is effected by increased salinity.
Steps:
1.) Go to the different stages in the classroom and complete Table 1: Comparison of Embryological Features of a Developing Sea Star, Frog and Chick. Also complete Table 2: Comparison of Ecological Aspects in Starfish, Frogs and Chick Development. 2.)Make a hypothesis based on Research Paper on Zebra Fish and Increased Salinity on Embryonic Development. 3.) Obtain two petri dishes, one with 3% NaCl and the other with Water. 4.) Select 40 healthy embryos and place 20 in each container. Stage at least 10 embryos in both the control and the 3% NaCl petri dishes. After Staging the Embryos Complete the chart for Day 1 noting the initial conditions of the embryos.
Observation:
Hypothesis: Zebra Fish are fresh water fish, so as the salinity increases there is a decrease in the development of the embryos.
The First Day we obtained 40 alive healthy Zebra Fish and Placed them into the Water (Control) and the 3% NaCl. The initial assessment and staging of 10 embryos of these fish was completed on day one (Table 1). On Day 3, the second assessment was completed on the fish, unfortunately all of the fish in the 3% NaCl were dead at this point. The control were still assess and staged (Table 1). This did validate the research paper as it stated that in early development the zebra embryos did not live past 2 hours in 4% NaCl. At this time, serial dilutions were made and new embryos were selected, this time 10 in each petri dish. Now there are 4 dishes, Control, 3% NaCl (new Control), 0.3% NaCl and 0.03% NaCl. From these dilutions one can assess the long term effects on the salinity with increased development. Table 2 shows the initial assessments of the new fish as well as the staging of 5 of them each. Again Monday the fish were reassessed and staged (Table 2). As expected the fish in the 3% NaCl were dead, showing that this was not a random error that occurred.
| ' | Day 1 (Wednesday) | ' | Day 3 (Friday) | ' |
| Control | 3% NaCl | Control | 3% NaCl | |
| Hatching: | 0 | 0 | 8 | 0 |
| Dead: Alive | 20 alive | 20 alive | 20 alive | 20 dead |
| Movement: | 1 | 0 | 10 | 0 |
| Yolk Absorption: | 0 | 0 | 0 | 0 |
| Eye Pigment: | 0 | 0 | black | 0 |
| Observe devemopment of body and Tail: | 0 | 0 | 0 | 0 |
| Heart Rate | - | - | - | - |
| Observe Tail/Fin Structures: | 0 | 0 | tails present after hatching | 0 |
| Measure Tail length: | 0 | 0 | 1-2mm | 0 |
| Staging: | ||||
| 1 | Germ Ring | 6 Hr Somite | Pec Fin | N/A |
| 2 | 14 Somite | 10-Somite | Pec Fin | N/A |
| 3 | 14 Somite | 6 Hr Somite | 21 Somite | N/A |
| 4 | 14 Somite | Bud-10 hr | 18 Somite | N/A |
| 5 | Bud 10 Hr | 6 hr shield | 21 Somite | N/A |
| 6 | Dome 4.3 Hr | 6 hr shield | 26 somite | N/A |
| 7 | 3 Somite | 4.7 hr Epiboly | 21 Somite | N/A |
| 8 | Epiboly 4.7Hr | Germ Ring 5.7hr | 26 Somite | N/A |
| 9 | 10 Hr Bud | Dome 4.3hr | 21 Somite | N/A |
| 10 | Germ Ring 5.7hr | Epiboly 5.3 hr. | Pec Fin | N/A |
| ' | Day 3 (Friday) New Concentrations | ' | ' | Day 5 ( Monday) | ' | ' | ' |
| 3% NaCl | 0.3% NaCl | 0.03% NaCl | Control | 3% NaCl | 0.3% NaCl | 0.03% NaCl | |
| Hatching: | 0 | 0 | 0 | 20 | 0 | 8 | 0 |
| Dead: Alive | 10 alive | 10 alive | 10 alive | 20 alive | 10 dead | 1 dead; 9 alive | 10 alive |
| Movement: | 1 | 0 | 0 | 20 | 0 | 9 | 8 |
| Yolk Absorption: | 0 | 0 | 0 | very little yolk absorbed | 0 | very little yolk absorbed | very little yolk absorbed |
| Eye Pigment: | 0 | 0 | 0 | brown | 0 | brown | brown |
| Observe devemopment of body and Tail: | 0 | 0 | 0 | Quick movement | 0 | Quick movement | Quick Movement |
| Heart Rate | - | - | - | - | - | - | - |
| Observe Tail/Fin Structures: | 0 | 0 | 0 | tails present,melanophores | 0 | tails present,melanophores | tails present,melanophores |
| Measure Tail length: | 0 | 0 | 0 | 2mm | 0 | 2mm | 2mm |
| Staging: | |||||||
| 1 | High Pec | 10 Somite | 14 Somite | Pec Fin | N/A | Long Pec | Pec Fin |
| 2 | High Pec | 18 Somite | 18 Somite | Pec Fin | N/A | Pec Fin | Protruding Mouth |
| 3 | 16 Somite | 18 Somite | 21 Somite | Long Pec | N/A | Protruding Mouth | 18 Somite |
| 4 | 18 Somite | 10 Somite | 10 Somite | Protruding mouth | N/A | Long Pec | High Pec |
| 5 | 10 Somite | 18 Somite | 14 Somite | Protruding mouth | N/A | Long Pec | Pec Fin |
| 6 | Pec Fin | ||||||
| 7 | Pec Fin | ||||||
| 8 | Protruding mouth | ||||||
| 9 | High Pec | ||||||
| 10 | Pec Fin | ||||||
Day 7-14 The water was continuously changed, and the zebra fish fixing and sac started for research. Most of the control started to die. This finding could have been from the snows storm and drying out of some water and lack of water change or lack of nutrients from the yolk. The tables below is the data collected these days.
| ' | Day 7 (Wednesday) | ' | ' | Day 9 (Friday) | ' | ' |
| Control | 0.3% NaCl | 0.03NaCl | Control | 0.03% NaCl | 0.3% NaCl | |
| Hatching: | 20 | 9 | 10 | all | all | all |
| Dead: Alive | 20 alive | 5 alive | 9 alive 1 dead | 18 | 5 alive 2 dead | 3 alive |
| Movement: | 20 | 5 (very little) | 9 | 18 | 5 | 2 |
| Yolk Absorption: | most of yolk absorbed | very little yolk absorbed | most of yolk absorbed | almost all | almost all | very little yolk absorbed |
| Eye Pigment: | Black | Black | Black | green | black | black |
| Observe devemopment of body and Tail: | Quick Movement | Tail flutter/ very slow | Quick Movement | purposeful | purposeful | tail flutter/very slow |
| Heart Rate | 140 bpm | 80 bpm | 120 bpm | 140 bpm | 110 bpm | 80 bpm |
| Observe Tail/Fin Structures: | tails present,melanophores | tails present,melanophores | tails present,melanophores | tails present,melanophores | tails present,melanophores | tails present,melanophores |
| Measure Tail length: | 2mm | 2mm | 2mm | >2mm | >2mm | 2mm |
| Day 7 (Wednesday) | Day 14 (Wednesday) | |||||
| Protruding Mouth | long pec | Protruding Mouth | Protruding Mouth | Protruding Mouth | High pec | |
| Protruding Mouth | long pec | Protruding Mouth | Protruding Mouth | Protruding Mouth | High pec | |
| Protruding Mouth | 18 Somite | Protruding Mouth | Protruding Mouth | Protruding Mouth | Long pec | |
| Protruding Mouth | 18 Somite | Protruding Mouth | Protruding Mouth | Protruding Mouth | ||
| Protruding Mouth | long pec | Protruding Mouth | Protruding Mouth | Protruding Mouth | ||
| ' | Day 13 (Tuesday) | ' | Day 14 (Wednesday) | ' |
| Control | 0.03% NaCl | Control | 0.03%NaCl | |
| Hatching: | 14 | 5 | 5 | 3 |
| Dead: Alive | 5 alive 9 dead | 3 alive 2 dead | 1 alive 4 dead | 1 alive 2 dead |
| Movement: | 5 | 3 | 1 | 1 |
| Yolk Absorption: | almost all | almost all | almost all | almost all |
| Eye Pigment: | Black/green | black | green/black | black |
| Observe devemopment of body and Tail: | purposeful | purposeful | very little | very little |
| Heart Rate | - | - | 100 | 80 |
| Observe Tail/Fin Structures: | tails present,melanophores | tails present,melanophores | tails present,melanophores | tails present,melanophores |
| Measure Tail length: | >2mm | >2mm | >2mm | >2mm |
| Staging: | Protruding mouth | Protruding mouth | Protruding mouth | Protruding mouth |
| Protruding mouth | Protruding mouth | |||
| Protruding mouth | Protruding mouth | |||
| Protruding mouth | ||||
| Protruding mouth | ||||
After Data was collected for the 14 days, the fixed zebra fish were examined to compare the 0.3%NaCl and 0.03% NaCl to the control to see what in the development stages were affected.
| ' | 26-Feb | ' | ' | ' | ' | ' | Dead |
| 0.03%-1 | 0.03%-2 | 0.3%-1 | 0.3%-2 | Control-1 | Control-2 | 0.3%-1-4 | |
| Fish length (micrometers | 2500 | 2500 | 2000 | 2000 | 2625 | 2625 | 1250 |
| Eye size (Micrometers | 250 | 250 | 200 | 200 | 275 | 275 | 250 |
| ' | 28-Feb | ' | ' | ' | ' | ' |
| Control-1 | Control-2 | Control-3 | 0.3%-1 | 0.3%-2 | 0.3%-3 | |
| Fish length (micrometers | 2625 | 2625 | 2625 | 1250 | 1250 | 1250 |
| Eye size (Micrometers | 375 | 375 | 375 | 225 | 225 | 225 |
| ' | 4-Mar | ' |
| 0.03% | Control | |
| Fish length (micrometers | 2625 | 2500 |
| Eye size (Micrometers | 225 | 250 |
Future: The Zebra Fish will have continued observation and eventually preservation to look why they have decreased development and effects that the salinity had in comparison to the controls.
3/6/2014
Question/Objective
To be able to use the DNA sequencing data to be able to identify the types of bacteria that area present in the pine transect.
Methods
Please refer to the DNA sequencing lab.
Results"
The DNA that was sequenced for our transect was unable to be sequenced. Luckily, there was other lab sections who also had this exact pine transect and were able to get a DNA sequence for their dilutions. 1.) Chyseobacterium which has a yellow/orange glossy appearance in a perfectly circular colony. The cells are gram negative rod shaped with a 0.5 µm in diameter. When culturing out bacteria we had a colony that fit this description. This bacteria is found in the soil, plants and various water sources. 2.) Streptomyces is an Acetinobacteria that is gram positive aerobic rod shaped bacteria. On the agar plate it appears to have a large white course appearance with indentations. There was no growth on out agar plates that had this type of bacteria present. Although this was a very large transect, and there are many more bacteria present that what we could have found (Streptomyces, 2010).
Work Cited:
Streptomyces. 2010. Bacteria in Photos. 3 March 2014. <http://www.bacteriainphotos.com/Streptomyces%20images.html>
2/26/2014
Question/Objective: To understand the more complex body plans and systems of invertebrates. Also, be able to identify invertebrates that live in the transect.
Steps:
1.) Given fixed slides/live specimens, observe the movement and body structures of acoelomates, pseudocoelomates and coelomates. 2.) Last week the Berlese funnel was set up with 50:50 ethanol/water underneath it. The invertebrates have fallen out of the soil into this solution. Obtain two petri dishes and pour off the top solution into one and the bottom solution (perhaps with some soil) into the other. 3.) Using a dissecting microscope, look though the solution and soil for species that resemble invertebrates. 4.) Select 5 organisms from this sample and complete table one, including the type of organism found, the length and description. If 5 are not present, observe other invertebrates from West Virginia. Use Dichotomous key for identification of major insects. 5.)Go over to the next section to observe arthropods that have been preserved for observation in jars. Complete The Student Hand out sheet with the correct identification of the arthropods according to description and what is observed.
Observation:
Observing acoelomates, Pseudocoelomates and Coelomates: The movement of these species are very different because of the different in the layers that are present in these organisms. First looking at the acoelomate planaria move in a wave-like motion. They are flat and symmetric with a digestive system. The second pseudocoelomates was a nematode. These were observed under a microscope and had very squirmy bead bobbing movements (they were all clumped together because there were so many, and lots of moving heads were seen.)These organism have all three tissue layers the ectoderm, endoderm and mesoderm. The mesoderm or muscular layer is responsible for the head moving and squirming that there are capable of. Finally, the Annelida which was the earthworm which no microscope was needed. This organism has all three tissue layers as well and it was evident watching it contract and extend its body with the muscle layers for movement. These three organisms had very different movements that differed because of their body structures and systems.
Invertebrate Collection Analysis: As one can see from Table 1, there were four invertebrates that were collected from the Berlese funnel. There was a large range in the sizes of the invertebrates from 0.2 mm-8mm (ranges given in table 1). The organism that was found that was the largest was the fly which was probably about 8mm in size. The dissecting microscope was still used to view the details of the organism. The smallest organism that was found in the transect was the soil mite at 0.2 mm. There are many organisms that are commonly found in a leaf litter. These invertebrates live in the leaf litters as a food source and break up the fungi and bacteria which can actually be beneficial to the plants (Lin, 2012). Some of the common ones are soil mites, earthworms, ground spiders, centipedes, millipedes and beetle larva (Bentley, 2014).
| Kind of Organism (Phylum) | Length in mm | Brief Description |
| Soil Mite (Acari) | 0.2-0.3mm | Round body, required magnification for visibility, pinchers bilateral, brown in color about 0.2mm |
| Flies (Diptera) | 8-12mm | Bilateral wings- translucent, stripes of colors across body (reddish/brown), bilateral eyes black in color |
| Ants (Hymengtera) | 6-7mm | parasitic, hard body, no wings noted, body was not compressed and was red in color. |
| Flat worm (Platyhelminthes) | 0.8-1mm | flat, white/clearish color, appeared to be symmetric |
| ***Biting Lice (Malloghaga) | 1-4mm | moth parts, parasite, brown/greenish in color. |
| *** This was a sample from West Virginia not the Transect | ||
Table 1: Identification of Five Invertebrates from Transect
| Does it have wings? | Yes | No | Yes | No | No |
| How many? | 2 | - | 2 pairs | - | - |
| Number of Antennae: | 2 | 2 | no | No | 1 pair |
| Number of Legs: | 6 | more than 10 | 8 | 10 | >10 |
| Number of Body Parts/Segments: | 3 | flat- many little | 2 | 2 | many |
| Which class does it belong? | Insects | Centipede | Crustaceans | Arachnids | Millipedes |
Table 2: Classification of Unknown Arthropods
Vertebrates and Niches:
After looking at invertebrates in the transect, one can only wonder what other kind of species live or pass through this transect on a daily basis. There are five that could live in this transect. See Table 3 detail about species and reasons for interest in this transect.
| Common Name | Phylum | Class | Order | Family | Genus | Species | Biotic/Abiotic Characteristics that would benefit the species |
| Deer | Chordata | Mammalia | Artiodactyla | Cervidae | Odocoileus | O. virginianus | food: plants/leaves, dark shelter |
| Northern Cardinal | Chordata | Aves | Passeriformes | Cardinalidae | Cardinalis | C. cardinalis | food-berries, insects, seeds |
| Pine Warbler | Chordata | Aves | Passeriformes | Parulidae | Setophaga | S. pinus | food-small invertebrates, berries |
| Eastern Gray Squirrel | Chordata | Mammalia | Rodentia | Sciuridae | Sciurus | S. Carolinensis | food- nuts and berries |
| Mole Kingsnake | Chordata | Vertebrata | Reptilia | Colubridae | Lampropeltini | L. calligaster | Food: rodents, insects |
Table 3: Vertebrate Species Who Could Live in Transect
Figure 1: Food Web This is a Food web for the vertebrates in my transect. The Snakes eat the birds and rats. The Birds eat earthworms which eat bacteria which feast on dead holly leaves. The Rats eat spiders which eat live leaves. The Deer are vegetarian and eat lives leaves and the squirrels eat nuts.
Future: Next we will look at the development or embryology of vertebrates.
Reference: Lin, K. "Seasonal Science: What Lurks in the Leaf Litter." Scientific American. October, 2012. <http://www.scientificamerican.com/article/bring-science-home-leaf-litter-biodiversity/>
2/22/2014 Question/Objective: Looking at Plants and Fungi, understand the characteristics of plants and what makes them diverse. Understand the functions and importance of fungi.
Steps: 1.) First obtain PCR specimen from previous lab and (after practicing) run the two specimens in an electrophoresis gel to see if the 16S rRNA gene has been copied. 2.)With three bags, return to the transect on Massachusetts Avenue and obtain more specimen samples. In one bag fill with soil to the top and in the second bag collect 5 plants from various locations in the transect. Make sure that these five are representative of the transect area. 3.) Lay out the leaves on the lab counter and Complete table one in the lab manual identifying the type of leaves, description, vascularization, leaves/special characteristics and seeds/reproduction. 4.) Complete the stations: Examine the slide of a lily plant, making sure to identify the xylem and phloem layers. Examine the leaves of moss and angiosperms. Make sure to compare all three observations. 5.) Observe and stage the PCR reaction for the two specimens that were placed. Giving any of the ones what reacted to the professor for refrigeration. 6.) Finally, using the bag of soil to set up the invertebrates collection experiment using a berlese funnel. Obtaining a bottle and fill with 35-40ml of 50:50 ethanol/water mixture. Hook the berlese funnel up with a clamp to a rod and place the bottle underneath the funnel. With the netting, create a filter by taping it in the funnel. Placing larger leaves down first, pour the soil into the flask. Tape to secure the apparatus.
Observations:
From the Transect, 5 plants were collected which were a representation of the vegetation from this area. The transect was very wet, because it was actively raining as the specimens were gathered. The 5 leaves that were collected were Ivy, Oak, Pine, Holly, and Maple. Picture 1 is visual of the leaves which were collected.
Picture 1: (from left to right) Ivy leaf, Oak Leaf, Pine needles, Holly leaves and a maple leaf.
The leaves were collected from different areas in the transect. Picture 2 is a visual of the location which each leaf was collected.
Picture 2: Location of the leaves which were collected. 1.) Ivy leaf; 2.)Oak Leaf; 3.) Pine needles; 4.)Holly; 5.)Maple leaf
After Identifying these leaves it was then that Table 1 was completed describe and characterize the 5 different plants collected from the transect.
Table 1: Transect Plants
| Transect | Location and # of Intrasect | Description | Vascularization | Leaves and Special Characteristics | Seeds, Evidence or reproductie starts |
| Ivy: genus: Hedera | 1 | Green, lvy leaf, long stem 6cm | roots, xylem, phloem | leaves, cuticle, stomata | dicot |
| Black Oak: genus: Quercus | 2 | brown oak leaf, appearing dead, 11cm | roots, xylem, phloem | leaves, cuticle, stomata | dicot |
| Waster Whole Pine: genus: pinus | 3 | pine, compound with scattered seeds, 12 cm | roots, xylem, phloem | Stomata, Cuticle, needle like leaves | seeds, gymnosperm |
| Holly: genus: Ilex | 4 | 13 holly leaves with 2 green berries, 5cm | roots, xylem, phloem | leaves, cuticle, stomata | dicot |
| Sugar Maple: genus: Acer | 5 | 1 brown maple leaf, 14 cm | roots, xylem, phloem | leaves, cuticle, stomata | dicot |
Plant Vascularization: All the leaves that were collected in this transect had the sample vascularization. They all had roots, xylem and phloem. The roots very important in the evolution of the plants when they were moved to the land to bring water and nutrients from the ground to the plants. There are two structures that are essential for the dispersion of water and nutrients to the whole plant, they are xylem and phloem. Xylem is dead tissue that moves water and dissolved minerals through the tracheids and vessel elements. This water is moved from high concentration to low concentration through the process of osmosis. The phloem is live tissue that uses energy to move nutrients through out the plants, utilizing four types of cells (Freeman, 2010).
Plant Specialization: The Ivy, Oak, Holly, Pine and Maple leaves all had a cuticle and stomata on their leaves. With evolution of the plants there were certain features that were essential to prevent the drying out of the leaves. The cuticle is the waxy coating on the leaves which one can visibly see in a nourished leaf. This is a protective layer for the leaves to allow them to hold in the water and prevent drying out. The stomata are a very important part of the leaves as they assist in the respiratory gas exchange in the plant. The stomata can be easily visualized under a microscope and look like eyes. Stomata are located on the underside of the leaves and have an opening in the center with guard cells on either sides to control the opening and closing of the stomata. The stomata have the ability to open and close in order to control the gas exchange and to hold in gasses as needed at night when no sunlight(Freeman, 2010). Even the pine needles which have a different shape than the other leaves also have the waxy cuticle present with the microscopic stomata for water vapor and gas exchange (Pines, 2014).
Plant Reproduction: The Ivy, Oak, Holly and Maple were all identified as dicots. The only one which was different was the pine. The pines has seeds and were gymnosperm.
Fungus: Fungi are spore producing structures called sporangia. This means that they are able to perform meiosis outside of the structure along with spore production (Freeman, 2010). Being a sporangia is important to fungus because the spores will grow into the adult and does not require host. Spores are released and an adult is formed without fusing with another cell, hence it is a diploid phase of the life cycle (Freeman, 2010). Fungi are so important for the environment as are consumers of living and nonliving organic matter and important for the survival of the eco life form such as the environment in our transect. A rhizopus sporangia zygote was examined under the microscope in lab, Picture 3 shows what was observed. This is a great example of a fungus because it has many features which distringusih it as a fungi. Namely, the sporangia which are the darkened areas in the center. There is also presence of the rhizoids with are the root like structures that are branching.
Picture 3: Fungus: Rhizopus Sporangia Zygotes at the 10x magnification
After observing the fungus, the Electrolysis was then reexamined. Group 5's hay infusion 10^-4 dulition showed positive results at about 3-400. 10^-5 there was no reaction. Below is the picture of the results in comparison to the control. Our results are chamber 5 below the numbers next to the control. (with the two positives in a row). This dilution was given to Alyssa to refrigerate.
Picture 4: Electrolysis Gel results for the PCR of hay infusion.
Future plans: Observe the invertebrates from the transect.
2/16/2014 Map of Transect:
1.) Hay Infusion Sample was obtained. 2.)Invertebrates Sample was obtained.
2/16/2014
Question/Objective: Looking at hay infusion bacteria, which was cultured on nutrient and tetracycline plates note characteristics of the different bacteria. Selecting three colonies perform wet mount for cell observation, gram straining and PCR.
Steps:
1.) Check on Hay Infusion and make observations on the changes that may have occurred over the week.
2.) Obtain the 7 cultures that were plated last lab and Complete Data Table 1 100-fold Serial Dilutions Results. Counting the number of colonies that have grown on the nutrient and tetracycline plates.
3.) Pick three of these plates at least one tetracycline and fill in data on Data Table 2.
4.) Note the size and characteristics of one colony on each plate.
5.) Wet mount a tiny bit of each of these three colonies mixing it with a drop of oil then placing a cover slip on top. Observe the sample under the microscope at 10x 40x and 100x. Note the cell descriptions of the cells including their shapes and arrangement.
6.) Perform gram staining. Take another tiny sample three bacteria colonies and again on three separate slides. The sample needs to be dried, so no water or cover slip is added, each sample is passed through a flame about 8-9 times or until appearing dry. Then each sample is washed with 1 minute of crystal violet, rinsed with bottled water, Gram’s iodine mordant for 1 minute, rinse with water, 95% alcohol for decolorizing for 10-20 seconds, washed with safranin stain for 20-30 seconds. Finally the samples are rinsed with water again and carefully dried before examination. Observe to identify all three sample as gram positive or negative.
7.) Perform PCR on a sample of the hay infusion for examination in next class. Mixing tiny amount of hay infusion sample with 100 microliters of water. Place this on a heat block for 10 minutes. After using the centrifuge, the sample is spin for 1 minute. With the DNA in the supernatant, pipette out 2 microliters of the DNA and mix with 23 microliters of the reverse primers. Place initials on the tiny tube and place into the PCR machine.
Observations: Hay Infusion observations: Upon analysis of the hay infusion this week it was noted that there were some changes to the environment. There was no film of algae at the top of the water as there previously was. Also, the potent smell that it had last week has decreased significantly. There was notable decrease in the water and the water was actually clearer with increased sediment on the bottom. These changes are due to the lack of nutrients that there is after one week. There is a disappearance of certain organisms due to the lack of energy required to sustain life, thus changes such as the smell decrease as well and the water being more clear.
Serial Dilution Results: Table 1 100 Fold Serial Dilution shows the colonies counted and colonies per milliliter results from the 4 nutrient and 3 tetracycline treated plates. Its was obvious to observe that the greater the dilution, for both the nutrient and tetracycline treated plates, the less colonies that grew on the plate. It was also noticed that the tetracycline treated plates showed significantly decrease in colony growths but also were larger in size when comparing to the nutrient plates. This tells us that the nutrient plates, had more colonies and they were smaller in size because they were growing on top of one another. Being that tetracycline is an antibiotic, the only growths on these three plates were those, which were antibiotic resistant to tetracycline. Tetracycline is a board spectrum antibiotic like many used for both gram-positive and gram-negative bacteria. The antibiotic affects the protein synthesis within the bacteria. So there were few growths of bacteria on these plates and no fungus present. It is known that there are approximately 29 different genes that are unaffected by tetracycline (Chopra & Roberts, 2001).
| Dilution | Agar | Colonies Counted | Conversion Factor | Colonies/ml |
| 10^-3 | Nutrient | 500 | x10^3 | 500x10^3 |
| 10^-5 | Nutrient | 100 | x10^5 | 100X10^5 |
| 10^-7 | Nutrient | 1 | x10^7 | 1X10^7 |
| 10^-9 | Nutrient | 0 | x10^9 | 10^9 |
| 10^-3 | Nutrient +Tet | 44 | x10^3 | 44X10^3 |
| 10^-5 | Nutrient +Tet | 1 | x10^5 | 1X10^5 |
| 10^-7 | Nutrient +Tet | 0 | x10^7 | 0 |
Bacteria Cell Morphology Observations:
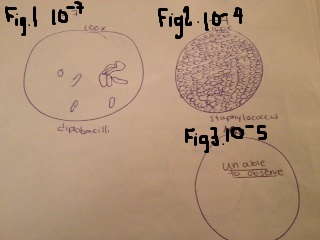
The samples that were chosen for further observation were plates 10^-5 (nutrient), 10^-7 (nutrient) and 10^-4 (tet). First the samples were placed onto a slide with oil and observed at 100x. It was obvious that plate 10^-7 (Figure 1) had the rod shape and was classified as a diplobacilli. Looking at 10^-4 (figure 2) there were hundreds of cocci also noted and was classified as staphylococcus. The last one observed, 10^-5 (figure 3), was unable to be classified under the microscope. There was a 3D appearance to this sample and even after getting another sample and staining, it was still not visible under the microscope. The professor was notified and stated that we might be able to visualize the shape after staining.
Figure 1 (top left): Observed at 100x magnification and was identified as a diplobacilli. Figure 2 (top right): Observed at 100x magnification and identified as a staphylococcus. Figure 3 (bottom right) "unable to observe" was being observed at 40x magnification and was not able to be observed even after straining.
Gram Stain Observations: The Gram staining is the use of crystal violet to see if the cell walls have a thick layer with peptidoglycan. If the cell is darkened only on the outside it means that this thickened membrane has taken up this dye into its cell membrane and is positive. If the cell appears all dark, this means that there is a phospholipid layer to the membrane which prevents this reuptake of crystal violet. Upon looking at our three samples it was observed that 10^-5 was gram positive because the hundreds cocci cells only had a darkened outside of the circle with clear on the inside. For sample 10^-7 and 10-4, the rods and cocci both appeared all dark indicating that they are gram negative.
Figure 4: (top left) Plate 10^-5, observed at 40x magnification, was gram Positive. The circles were very apparent, stain in the cell wall.(Top right) Plate 10^-7, observed at 40x magnification, was gram Negative. Rods filled with stain. (Bottom left) Plate 10^-4, observed at 10x magnification, was gran Negative.
Finally, this last image is the spreadsheet of all these results.
result
| Label Colony | Tet or Nutrient | Colony Description | # colonies | Cell Description | Gram Positive or Negative |
| 10^-5 | nutrient | yellow, glossy/slimy appearance, perfectly round, about 5mm | 100x10^5 | staphylococcus, clustered all together | Gram positive |
| 10^-7 | nutrient | yellow/white, on side of the dish, blob/oval shape, about 20mm | 1x10^7 | diplobacilli, rod shaped with some alone and some clustered, no motility | Gram negative |
| 10^-4 | Tet | white, perfectly circular, glossy appearance, about 10mm | 1x10^5 | staphylococcus, hundreds of circular, all attaching to one another, no motility | Gram negative |
Future Plans: Electrophoresis will be run on the PCR results and the plants and fungi of the hay infusion will be looked at.
Work cited: Chopra, I. & Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. June 2001. National Institute of Health. 15 February 2014. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC99026/>.
2/6/14 lab 1 notes
Great job! Start working on building a map of your transect to detail your land and where your samples are taken from. We will talk about this more Wednesday. Great work!
AP
January 22, 2014 Day 2: Hay Infusion Culture Observations: Question/Objective: With the understanding of a dichotomous key be able to identify protists from ones niche.
Steps: 1) Looking at the Hay Infusion write down the changes that have occurred over the last week. 2) Take three samples from the hay infusion and put them on a slide with a coverslip 3) Using the dichotomous key identify 6 protist that were observed on the slide. 4) Draw pictures of these organism that were found and obtain measurements and characteristics. 5) Prepare serial dilutions: Gather 4 test tubes labeling even them 2-8. Place 10 ml in each tube. Using a 100 microliter pipette put 100 microliters of hay infusion sample into the 1st test tube. Mix this solution then take out 100 microliters and place this into the 2nd test tube. Repeat until test tube 4. 6) Obtaining 4 nutrient agar plates from the instructor label each –3, -5, -7,-9. Then obtain 3 nutrient agar plates with tetracycline and label then –2,-4,-6. 7) Starting with test tube 8 pipette 100 microliters of sample onto the plate that is labeled –9 and spread the sample evenly around the plate, continue with tube 6 and plate –7 and so forth. 8) The same procedure for the plates with the tetracycline starting with plate –6 and sample from test tube 6. Continue for –4 from test tube 4 and –2 from test tube 2.
Observations/Data:
Hay Infusion Observations: Looking at the ecosystem that was created last class, it is easy to see that there have been a lot of changes in one week. Firstly, the smell, which was a dirt/soil smell, has changed to a potent/harsh moldy smell. Upon initial assessment of the jar, the water was much darker dirt brown with increased black sediment on the bottom of the jar. There was a clear film that was visible across the top of the water. There was new growth on some of the pine needles and leaves within the liquid. It is predicted that in two months there will be even more growth to the hay infusion. The hay infusion will continues to have growths of bacteria, protist and algae as the weeks go on as long as the temperature is held the same, adequate sunlight, food/energy and oxygen. The growth will plateau if any of these conditions change in the next two months. Inadequate temperature, sunlight, food/energy and oxygen would result in the selective pressures, which are pressures that cause the reproduction to decrease in the environment.
Sample Selection: When looking at the ecosystem with the group it was decided where the three samples needed to come from. The first was from the top, when taking this sample it was very difficult to penetrate the film that was on the top of the water. The second sample was from the middle around the needles and the third was from the bottom where there was a lot of darker sediment. These different areas should theoretically yield different types of species. One would expect that species that are living near other plants matter rely on those plants for energy. On the other hand, species on the top of the water are able to produce energy from other forms such as photosynthesis. Figure one: Hay Infusion Culture Observations:
In order for an organism to be considered alive they have to meet certain requirements. They must be able to obtain and utilize energy, be made up of cells, process information through DNA and RNA, have the ability to reproduce and of course come from other cells/being able to evolve (Freeman, 2010). Looking at the amoeba that was found in group 5’s hay infusion it is evident that it is a living thing and has all these qualities of living things. Amoebas obtain food by engulfing it (decaying vegetation), as it moves in a slow creeping way (Freeman, 2010). It is a protist that has a nucleus with genetic information and can be unicellular or multicellular. They are able to reproduce through the process of meiosis to form spores (Freeman, 2010). Amoebas have evolved from and there is many different types exemplifying that the species has been capable of evolution.
Figure 2: Serial Dilutions:
Future Plans: With the hay infusion solution plated on the agar, the bacterial will grow on the plates and will be examined next week.
Citation: Freeman, Scott. 2002. Biological Science. Prentice Hall: New Jersey. (2, 569-570).
January 22, 2014 Lab notebook and username entered successfully. -SMS
great work! MB
January 16, 2014 Day 1: Visit Transect to Study and Obtain Sample for Hay Infusion
Question/Objective: Given a 20x20 transect, analyze the land on the American University Campus and describe the general characteristics of the area including the biotic and abiotic components.
Steps: 1- TA walked group 5 to the transect area by the Church on Massachusetts Ave. 2- Having area cleared by Ben, a sample was collected from the transect. The sample included about 50% of the dirt/soil and the other the leaves/needles/vines and ivy. It was collected this sample in a 50ml conical tube using metal shovels. 3- Then 11.99g of the sample was measured out and placed into a labeled plastic jar, which had 500ml of water in it. 4- After adding 0.1g of dried milk to the sample in the water, it was gently mixed by swirling for approximately 10 seconds. 5- This transect was placed in the back of the room in the lab.
Observations: General: In the 20x20 section that group 5 was given was in a very dark shady area. The darkness was from the church and the trees in the area. Trees: There are three large trees in the area. The first one was a holly tree with scant amount of red berries on it and was healthy looking/damp from the previous days rainstorm. The second tree was the tallest which appeared to be dead. There was an area where the bark was missing; there was no leaves or evidence of recent leaves on this tree. The last tree is a pine tree with lots of pine needles still attached and healthy looking. This tree was also damp from the rain. Ground: On the ground there was lots of ivy that covered nearly the whole 20x20 area that was provided. There were lots of leaves that were from the trees, including the spiky brownish lifeless pine needles, maple leaves, acorns, and holly leaves. There were lots of small branches of each of the trees noted in this area as well. Under the ivy there was soil, mulch and vines from the trees and the ivy. There were no animals/insects noted in the area that were observed during this initial visit to the intersect. Abiotic: water, soil, air, and minerals in the dirt Biotic: trees, fungi on the tree trunks, leaves, ivy
Future Plans: The hay infusion, which was collected, will stay in the lab for another week. Observations will be documented after one week and prokaryotes will be examined from two locations under the microscope.-SMS