User:Tara K. Luckau/Notebook/Team ConGen/2011/07/07: Difference between revisions
From OpenWetWare
(Autocreate 2011/07/07 Entry for User:Tara_K._Luckau/Notebook/Team_ConGen) |
|||
| (4 intermediate revisions by the same user not shown) | |||
| Line 9: | Line 9: | ||
== | ==Data Confusion== | ||
* | * Some heavy confusion about what I'm looking at in the frag data. Today's been spent on the internets, figuring out what might be happening. I'm hesitant to go forward with PCRs until I understand a bit more what's going on. | ||
=== | ===The Evidence=== | ||
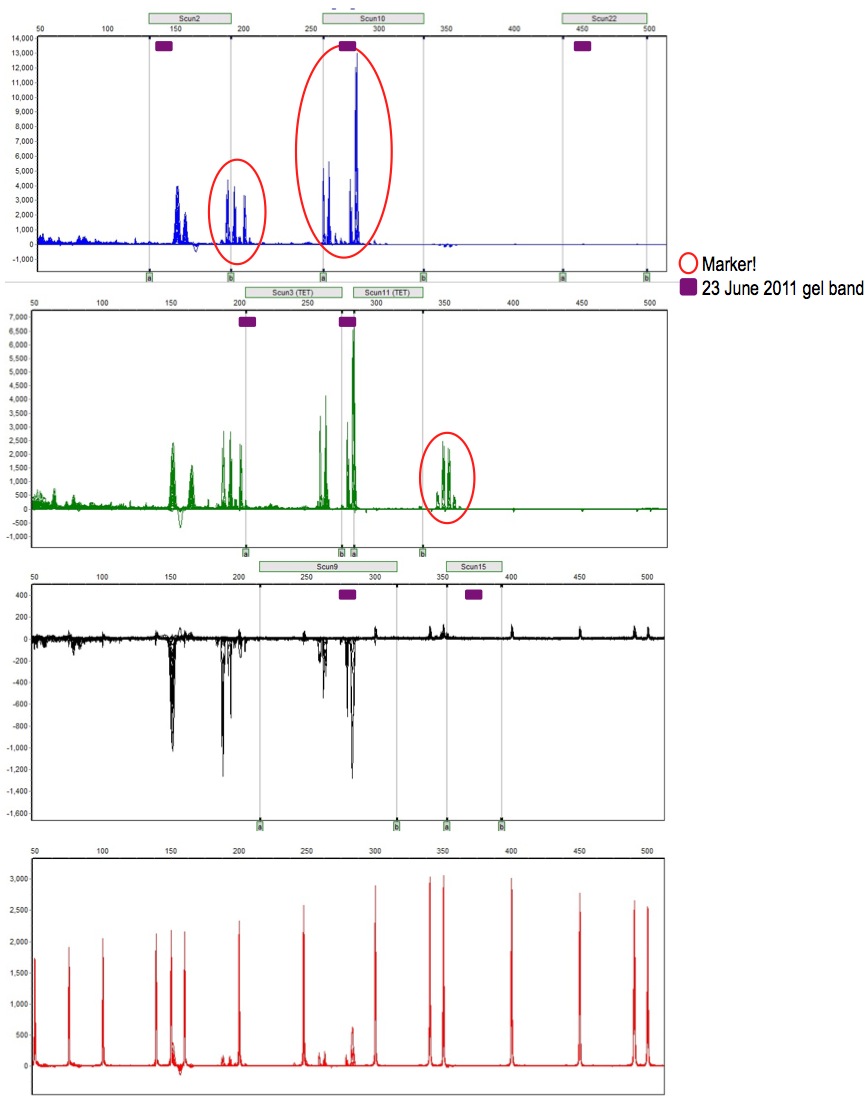
* here | * Okay, so below is shown three sets of data in one graphic: | ||
: Panel (gray rectangles) was set up based on the amplicon size ranges described in Lance et al. | |||
: Frag data (pherogram) shows 3 markers amplified to detectable levels (red circles) | |||
: Gel data (purple boxes) from uniplexes are slightly different amplicon sizes | |||
* Notice: | |||
: gel band size tends to be on the small end of expected amplicon size based on Lance's paper | |||
: frag peaks don't align with gel band size - difference in migration between agarose gel and capillary? | |||
: blue lane amplicons groups (Scun2 and Scun10) seem like they are likely, given the species difference | |||
::(primers developed on Sceloporus undulatus; amplified here on Sceloporus occidentalis) | |||
: green lane amplicons group is far outside the expected size range of Scun11 and far different from the agarose gel band size | |||
:: however, consistent with Scun15 (labeled with HEX) expectations and gel band | |||
* [[Image:20110707 DataConfusion.jpg|900 px]] | |||
===The Possible Explanation=== | |||
* The dye set I'm using (FAM-TET-HEX) is incompatible with UAGC's instrument set-up (filter set), which expects FAM-VIC-NED | |||
* HEX (what I want to be yellow) has the same optical spectral as VIC (what the instrument calls green) | |||
* thus, it's possible that the instrument didn't see anything labeled with TET, and interpreted those amplicons labeled with HEX to be green | |||
* This would produce a trace with amplicons in unexpected places in the green lane, and an empty yellow lane ... which is exactly what I got | |||
* [[Image:20110707 FluorophoreWhat?.jpg|800 px]] | |||
* the above is what I sent to Jon at UAGC - been communicating with him all day. He's not entirely sure, but says it might be possible to re-run my frag data, if that's what is required. | |||
<!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | ||
Revision as of 13:01, 8 July 2011
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |
|
Data Confusion
The Evidence
The Possible Explanation
| |