User:Torsten Waldminghaus/flow cytometry notes: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
| Line 54: | Line 54: | ||
#Add 250 μl freshly made FITC (3μg/ml in PB). Store in 4 °C '''over night'''. | #Add 250 μl freshly made FITC (3μg/ml in PB). Store in 4 °C '''over night'''. | ||
#Remove the FITC: Spin down the cells and wash in 1ml TBS pH 7.5, containing 130 mM NaCl (see above)(Include the 10*std) | #Remove the FITC: Spin down the cells and wash in 1ml TBS pH 7.5, containing 130 mM NaCl (see above)(Include the 10*std) | ||
#Resuspend samples and 1*st in 500 μl TBS and the 10*std in | #Resuspend samples and 1*st in 500 μl TBS and the 10*std in 100 μl. | ||
#Put 250 μl of the samples and 1*st into flow cytometry tubes | #Put 250 μl of the samples and 1*st into flow cytometry tubes | ||
#Add 250 μl of 3 μg/ml Hoechst 33258 (dissolved in TBS) and | #Add 250 μl of 3 μg/ml Hoechst 33258 (dissolved in TBS) and 100 μl to 10*std. | ||
#Let cells stain in dark on ice for at least 30 minutes (time to go to the flow cytometer and set it up is usually enough. | #Let cells stain in dark on ice for at least 30 minutes (time to go to the flow cytometer and set it up is usually enough. | ||
#Add about | #Add about 5 μl of the 10*std to each sample before running. | ||
*'''FITC solution''': 3 mg FITC is dissolved in 10 ml PB (gives 0.3 mg/ml). 100 μl of this stock is dissolved in 10 ml PB, wich gives 3 μg/ml (prepare fresh every time). | *'''FITC solution''': 3 mg FITC is dissolved in 10 ml PB (gives 0.3 mg/ml). 100 μl of this stock is dissolved in 10 ml PB, wich gives 3 μg/ml (prepare fresh every time). | ||
Revision as of 07:20, 10 January 2011
Recipe for flow-TBS
- 1x TBS with 20mM Tris used for flow cytometry in Skarstad-Lab (reduced NaCl concentration from 150mM in the usual TBS to 130mM (everything else is the same).
| for 500 ml |
|---|
| 10 ml 1M Tris-HCl pH 7.5 |
| 13 ml 5M NaCl |
| 477 ml ddH2O |
- Prepare and sterile filter. Store at 4°C.
Preparation of E. coli for flow cytometry
- Two kinds of samples are prepared:
- Exponential samples: directly from the culture (usually at OD=0.15) to messure whole DNA content.
- Rif/Cpx samples: Rifampicin stopps transcription and through this initiation of replication while elongation continues. Cephalexine stopps cell division. Combination of both antibiotics will lead to cells containing fully replicated chromosomes. The number of chromosomes corresponds to the number of origins per cell at the timepoint of drug treatment.
- After taking 1.5 ml exponential samples add 150 μg/ml rifampicin, and 10 μg/ml cephalexin to the culture (Stock solutions: Rif: 30 mg/ml and Cpx: 5 mg/ml)
- Continue to grow for 3-4 hrs., wash, fix.
Fixing the cells:
- Take 1.5 ml cell culture in an eppendorf tube and put on ice.
- Centrifuge at 15000 rpm / 4 min. at 4°C.
- Remove the supernatant, and resuspend the pellet in 1 ml filtered (filter through 0.22µm otherwise debris will give bakcground lightscatter) TE buffer.
- Spin as before and remove supernatant.
- Add 100μl TE and vortex to resuspend cells.
- Add 1 ml 77% filtrated ethanol.
- Store the samples at 4°C. Samples may be stored for several weeks.
Cell staining with FITC and Hoechst 33258
- Fluorescein isothiocyanate (FITC) stains proteins and gives messurement of the cell size
- Hoechst 33258 stains DNA
- All procedures are performed on ice
- Cells are washed in TE and fixed in ethanol (see above).
- Spin down cells. Include one tube of 10*std per 20 samples
- Wash the cells in 1 ml of 0.1M KH2PO4/K2HPO4 pH 9.0 (PB) (pH 9 is for optimal for FITC staining).
- Resuspend in 250 μl PB.
- Split the 10*std in 2:
- 25 μl plus 225 μl PB is treated like the samples (= 1*std)
- 225 μl is not stained with FITC but only Hoechst (= 10*std)
- Add 250 μl freshly made FITC (3μg/ml in PB). Store in 4 °C over night.
- Remove the FITC: Spin down the cells and wash in 1ml TBS pH 7.5, containing 130 mM NaCl (see above)(Include the 10*std)
- Resuspend samples and 1*st in 500 μl TBS and the 10*std in 100 μl.
- Put 250 μl of the samples and 1*st into flow cytometry tubes
- Add 250 μl of 3 μg/ml Hoechst 33258 (dissolved in TBS) and 100 μl to 10*std.
- Let cells stain in dark on ice for at least 30 minutes (time to go to the flow cytometer and set it up is usually enough.
- Add about 5 μl of the 10*std to each sample before running.
- FITC solution: 3 mg FITC is dissolved in 10 ml PB (gives 0.3 mg/ml). 100 μl of this stock is dissolved in 10 ml PB, wich gives 3 μg/ml (prepare fresh every time).
- Hoechst 33258: Stock solution is 3 mg/ml in TBS. Dillute 6 μl of stock in 2 ml TBS to get 3μg/ml solution. The dilluted Hoechst 33258 can be stored at 4°C .
- 10*std is a standard of cells with 1 and 2 chromosomes, 10-fold concentration
Use of flow cytometer BD LSR II
- When started new no washing is required but before starting with samples one should press 'Prime' without any liquid (never have 'Prime' with liquid!!!' to let run air run through the system. Than run water on setting "high" on the cytometer (press 'run').
- Start software FACSDiva:
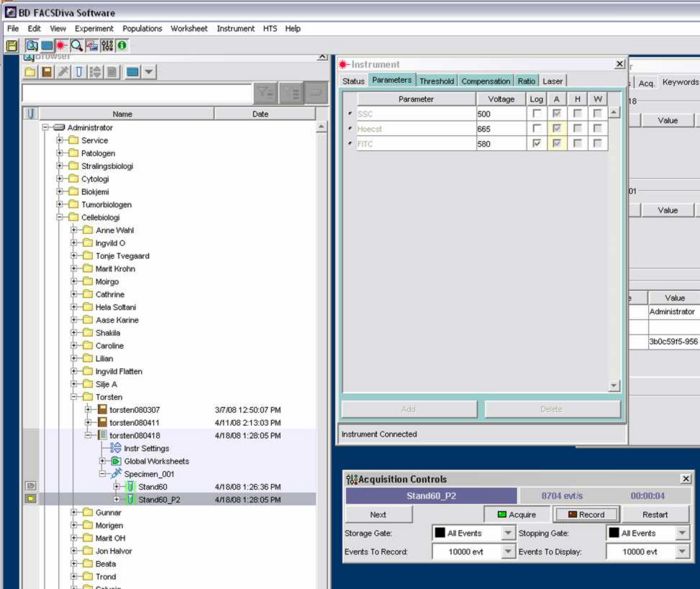
- Select Instrument Configuration
- Select presettings of Gunnar F
- Press 'Set configuration' and OK
- Go to Cellbiology > Torsten > new exp. (brown icon) > rename (torsten date)>
- press syringe icon
- go to 'tube_001' > things will show up on right screen (Without diagramms shown in picture. Those are set in the following):

- make local sheet by pressing on icon beside experiment (left screen)
- Create diagrams:
- Click on DotPlot icon and than on the sheet
- Axes can be changed by clicking on the axe-title (right click)
- Make dot plot with FITC versus Hoechst
- Make 4 histograms:
- Count/SSCA
- Count/Hoechst
- Count/FITC
- Count/Hoechst
- Go to Instruments (window on the right of the left screen) > Parameters > Voltage:
- SSC: 500
- Hoechst: adjust later using the standart /linear scale
- FITC: 580 /log scale
- Go to Instruments > Threshhold:
- Press 'Add' two times; delete SSC and change Hoechst to 5000 for Standard (for samples it should be 2000)
- Go to laser and set windows ext. to 2000
- UV delay is 40.000 and can be changed if one gets strange results. (To change, watch the FITC histogramm when delay is changed. The detected FITC should not go down when delay is changed)
Recording the standard:
- Press "low" at cytometer and insert standard cells
- In the Aquisition Controls window set the following:
- Storage Gate: All
- Events to record(number of cells counted): 10.000
- Number of events should be adjusted with the smal weel to about 1000-1500 (per second)
- The dot plot shows two populations: one with FITC staining and one without
- Lay two windows (vertical) over the dot plot to seperate the two populations (The corresponding name will be P1 and P2)
- Assign FITC-positive cells to one Hoechst-histogram and FITC-negative to the other (right click on diagramm headding > select show population P1/P2)
- Create statistic report by right clicking on the headding of the dot plot and selecting 'stat. view' from the appearing menu
- Adjust Hoechst voltage to fit one-chromosome peak of FITC negative cells to channel 60 (make a vertical window from 50 to 70 and monitor the report which gives the maximum that should be at 60)
=> The FITC positive cells show the peak than at about 40 (FITC quenches the Hoechst staining)
=> The numbers (volts, channel for - and + FITS) should be written in book
- Record 10.000 events by pressing 'Aquire' in the Acquisition window (left screen)
- Change 'Storage Gate' to P2 (FITC +) and record the standard again (for every new record press 'next' in the Acquisition window and give the new sample a suitable name)
- Change the Hoechst voltage to move the one chromosome peak to 15 (This is for fast growing cells to not miss high numbers of chromosomes)
=> Write down parameters (FITC+ should be arround 10.5)
- Record 10.000 events with 'Storage Gate' = All and than only P2
Messure samples:
- Exchange standard with first sample
- Change Hoechst Threshhold to 2000
- Click on 'Next' and rename (short name since WinMDI has problems with long names)
- Watch the event number (1000-1500)
- Move the peak of FITC - cells (standard) to 15
- Record P2 for 10.000 events
Export data:
- Export data as fcs 2.0 files (for WinMDI)to K:\Alle\KF_Flow\Montebello\Torsten Waldminghaus\
- Set the FITC data from log to linear
- Analyze data with WinMDI