20.109(F11): Mod 1 Day 7 FACS analysis
FACS analysis
Introduction
FACS stands for "Fluorescence Activated Cell Sorting." The FACS machine has revolutionized biology by allowing researchers to isolate cells based on their spectral qualities. For example, if you have a fluorescently tagged antibody that preferentially binds to a certain cell type, you can isolate a pure sample of this cell type from a complex mixture by using a FACS machine. In addition to purification, the FACS machine can count the number of cells that have a certain spectral quality. If a FACS machine is used just for counting and not for separating subpopulations of cells, then the procedure is called "flow cytometry," and this is what you will be doing today.
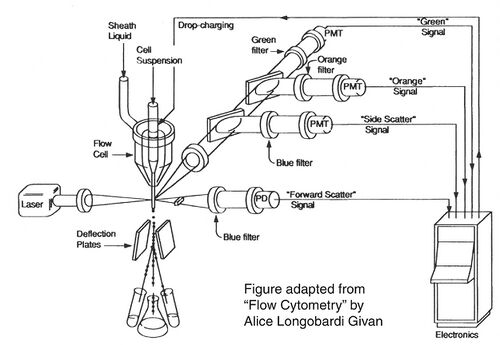
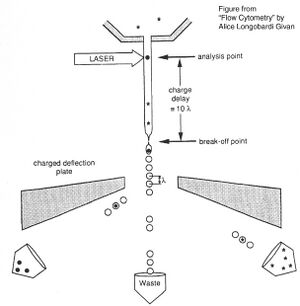
Before there were FACS machines, there were Coulter counters. Coulter counters are automated cell counting machines developed in the 1950s that count cells as they flow in a liquid stream. In an ingenious conceptual leap, Mack Fulwyler combined the technology of ink jet printers with that of Coulter counters to develop the first FACS machine. The ink jet printer head works by vibrating a nozzle so that a spray of discrete droplets is formed. Similarly, in a FACS machine, a liquid suspension of cells is forced at high pressure through a vibrating nozzle to create tiny charged droplets, each containing a single cell. The stream of droplets pass in front of a laser beam, and the scattered light is analyzed by a series of filters and photomultiplier tubes that convert the light signal into electrical impulses. Thus, each cell is "interrogated."
For FACS, the spectral qualities of the cell are analyzed nearly instantaneously and compared to your desired spectral qualities. For example, if you have a mixture of green fluorescent cells and non-fluorescent cells, you can ask the machine to isolate the green cells. If a cell registers as green, an electrical charge deflects the cell to make it fall into a collection chamber.
FACS is technically challenging and most FACS machines are only run by experts. In contrast, biologists are often trained to perform flow cytometry in order to analyze the proportion of their sample that has particular spectral qualities. You will be using flow cytometry to measure the percentage of cells that are fluorescent. You have lipofected cells with two non-functional EGFP genes. Recombination between these two genes can restore the full length EGFP coding sequence so that cells express EGFP. By measuring the percentage of cells that fluoresce green, you will have some measure of the frequency of homologous recombination within mammalian cells.
Protocol
Preparing your cells for FACS analysis
The following protocol should be performed in the sterile hoods unless otherwise indicated.
- Aspirate the media from your cells and wash them with 1 ml PBS, aliquotted with a 10 ml pipet.
- Add 200 μL trypsin to each well, aliquotted with a 2 ml pipet. After the last addition, start a 1’ timer. During this time, rock the plate in each direction to distribute the trypsin over the cells.
- Aspirate the trypsin and incubate the cells at 37°C for 10 minutes, using your timer to precisely time this incubation.
- Resuspend the cells in 100 μL OptiMEM, using your P200 to make an even suspension. Move each sample to a labeled FACS tube---pooling the triplicate samples to insure that you have enough cells for FACS.
- Keep your tubes on ice as you walk to the FACS facility.
FACS analysis of transfected cells
Carefully observe the TA run the flow cytometry machine. Be sure to ask questions if you don't understand the purpose of any step. Ultimately, you want to compare the percentage of green-fluorescent cells in each sample.
For next time
- Please submit your finished Mod 1 powerpoint summary. This assignment is due by 1PM on the Wednesday after the Columbus Day holiday. Please turn in your memo electronically by uploading it to the Stellar website that is associated with our class. It is important that you name your file according to this convention: Firstinitial_Lastname_LabSection_assignment.ppt, for example: S_Hockfield_TR_memo.ppt. There will be a 1/3 letter grade penalty for each day (24 hour period) late. If you are submitting your assignment after the due date, it must be emailed to bevin, nkuldell and astachow AT mit DOT edu. There will be no re-write option on this assignment.
- If you have not contributed your thoughts, comments and ideas to the 20.109 class blog, remember that you are required to post at least 300 words once each experimental module.
- Begin to familiarize yourself with the content of the second experimental module by reading the front page for the module as well as skimming the introduction to the first day of labwork.
