Isotachopherisis - Bridget Stanford, Josh Paine, Nicholas Mancini
Background
History
Isotachophoresis was first introduced as a method of separation in the late 1960s by Everaerts, stemming from knowledge of electrophoresis. Since then, the technology has been widely used and researched.[1] Today, it still remains one of the newest and least well understood electrophoretic methods. It reached peak popularity in the 1970s because it could be performed in large-bore capillaries in contrast to capillary zone electrophoresis which could not due to excessive joule heating. However with the introduction of narrow-bore fused-silica capillaries, it was all but replaced in favor of the newly introduced capillary zone electrophoresis.[2] Then in the 1990s there was a resurgence in the popularity isotachophorosis as there was a need for more sensitive preconcentration techniques. Isotachophoresis coupled with capillary zone electrophoresis massively increased the sensitivity and served to bring isotachophoresis out of its relative obscurity.[2]
Method
Basics
Isotachophoresis is a form of electrophoresis. By definition, electrophoresis is the movement of charged particles in a fluid or gel under the influence of an electric field. One of the most common types is gel electrophoresis, commonly used to separate DNA fragments by length. All forms of electrophoresis, including gel electrophoresis, take advantage of the difference in the electrophoretic mobility of charged particles. Isotachophoresis is different because it relies on the presence of a leading and terminating electrolyte buffer solutions to perform this separation. Using this process, anions or cations can be separated with very little band broadening due the electric fields generated by the buffers.[3] This is one important distinction that isotachophoresis has compared to ordinary electrophoresis, as running the separation for longer periods of time will not result in an increased degree of separation. It is important to note that isotachophoresis only separates a group of a single type of ions (i.e., either a group of anions or a group of cations) at a time and is unable to separate anions and cations from each other.
Like other forms of electrophoresis, isotachophoresis is a method of separation of ions that is used to isolate an ionic analyte. In doing so, a purified ion solution can be created if the technique is carried out properly. Further analysis or other use can then be carried out on the desired ion due to the higher purity of ion that can be created via this method.
Detailed Process
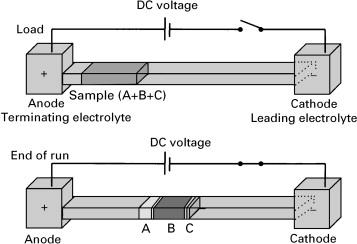
Isotachophoresis is executed in a discontinuous electrolyte system with two solutions, the leading electrolyte (LE) and terminating electrolyte (TE) solutions. These two solutions must be composed of ions of differing mobility. In each LE or TE solution, there are two types of ions; the co-ion and the counter-ion. The co-ion of the LE should be chosen such that it moves faster than the co-ion of the TE in the direction of migration of the analyte.[3] A common counter-ion should be chosen for both the LE and TE, such that electroneutrality is maintained. As an example, in an experiment designed to isolate a cationic analyte, a cationic co-ion of greater ionic mobility should be chosen for the LE, and a cationic co-ion of lesser ionic mobility should be chosen for the TE. The analyte sample is inserted between LE and TE solutions and an electric field is applied. When the electric field is applied it causes all ions to move towards the appropriate electrode, arranging themselves in order of mobility by moving at different velocities. Once at steady-state, all of the ions will migrate at the same velocity.[2] The leading and terminating electrolytes lock the analyte in place in between them and the electric field gradients generated by them prevent the diffusion of the analyte leading to highly concentrated samples.[3]
As a specific example of the experimental setup, in the case of isolating an anion from solution, one would typically choose chloride as the co-ion in the leading electrolyte, and glycine as the co-ion in the terminating electrolyte.[3] This specific orientation is chosen due to the very high mobility of chloride and the very low mobility of glycine in response to an electric field. As a rule of thumb, smaller ions will have a higher ionic mobility when compared to larger ions of the same charge.
Theory
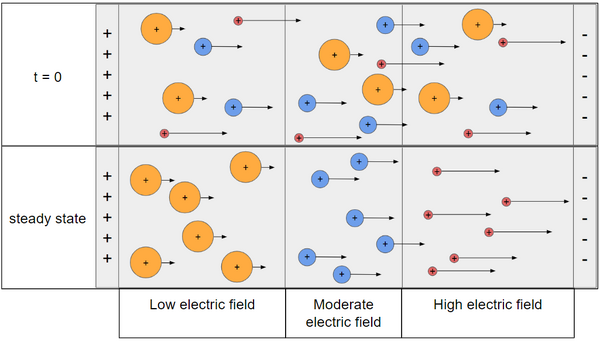
Charged analytes in solution have the ability to migrate when in the presence of an electric field. The isotachophoresis technique takes advantage of this principle by separating ions based on their ionic mobility of an ionic analyte, which is the movement of a charged particle in response to an electric field. Ionic mobility is calculated by the following general equation, with SI units of [math]\displaystyle{ {m^2 \over s \cdot V} }[/math]. [math]\displaystyle{ \mu = {v \over E} }[/math] with [math]\displaystyle{ \mu }[/math] as the ionic mobility, [math]\displaystyle{ v }[/math] as the ion velocity, and [math]\displaystyle{ E }[/math] as the electric field strength.[4] Figure 1 shows the movement of the different ions as isotachophoresis proceeds.
As the LE, TE, and analyte ions are subject to the electric field, the ions with higher ionic mobility (the leading electrolyte) will move faster than those with a lower ionic mobility (terminating electrolyte), with the analyte having an ionic mobility somewhere in between as these ions migrate at different velocities. Additionally, the migration of the three different ions causes the formation of three zones of differing electric field to form. This ensures that ions that drift into other zones will be forced back into their correct respective zone. Figure 2 shows the relative movement and zone creation that is utilized in this separation method.
Advantages
Isotachophoresis is very useful in analytical chemistry, with one of its major advantages being its high reproducibility. Isotachophoresis prevents diffusional broadening because the electric fields generated by the leading and terminating electrolytic solutions prevent diffusion.[3] Isotachophoresis can achieve million fold preconcentration and is ideal for use of analytes in complex matrices like blood, urine, or other biological fluids.[5] For example, nucleic acids have higher electrophoretic mobilities than similarly sized biomolecules such as proteins present in the matrix, making isotachophoresis ideal for use in such scenarios.[6] The sample preparation for isotachophoresis is extremely minimal along with the process itself being very simplistic.[6]
Disadvantages
The most notable disadvantage of isotachophoresis is that the leading and terminating electrolyte need to be carefully selected for optimal results.[5] Isotachophoresis has also faded in and out of obscurity and has only recently begun to see a major increase in popularity, meaning plenty of chromatographers are likely unfamiliar with it.[1] Additionally, care must be taken when attempting to separate an analyte from a solution containing ions of the same charge and ionic mobility. If this is the case, the final sample will contain all ions that have the same ionic mobility of the analyte which may be undesirable.
Applications
A specific example of where isotachophoresis is used is in in the separation of proteins. For many reasons, it can be desirable to purify a protein whether it be for the diagnosis of a disease, the creation of a drug, or for other research purposes. In each of these cases, isotachophoresis can be taken advantage of for these very purposes. Because many proteins carry an overall charge, they inherently have an electrophoretic mobility that dictates their movement in an electric field. Because proteins are typically very large on the molecular scale, their charge densities and electrophoretic mobility are likely unique to each protein. As such, isotachophoresis may be a very useful separation technique that requires minimal setup and equipment making it ideal for low-cost protein purification. Additionally, isotachophoresis allows for the separation of very similarly charged ions that may not be possible with traditional electrophoresis methods.
Microfluidic Isotachopherisis
Isotachophoresis first started being used on microfluidic devices in the early 1990s with the first formal report on such a device being released in 1998. However microfluidic devices were more challenging to use early on as there were less available techniques for creating them. Thus, as the popularity of microfluidic devices started to increase, so too did the use of isotachophoresis in microfluidic devices.[2] Due to the surge in popularity of microfluidics there have been broad implementations of isotachophoresis in microfluidic devices. Analytical chemistry is one the fields in which isotachophoresis sees the most use, as such many microfluidic devices for the purpose of analytical chemistry have been made that utilize isotachophoresis. For instance PDMS gel devices have been developed to separate small samples of metal cations in half the time it would have taken a capillary system.[2] This improvement in separation time is likely a product of transitioning to the micro-scale, where the diffusion of ions is observed to be faster at smaller scales. PDMS isotachophoresis devices were also used in the first fully polymeric microfluidic chip with electrodes made out of conductive polymers.[2]
References
1. Hirokawa, T. Electrophoresis | Isotachophoresis. In Encyclopedia of Separation Science; Elsevier, 2000; pp 1272–1280. https://doi.org/10.1016/B0-12-226770-2/03611-5.
2. Smejkal, P.; Bottenus, D.; Breadmore, M. C.; Guijt, R. M.; Ivory, C. F.; Foret, F.; Macka, M. Microfluidic Isotachophoresis: A Review. Electrophoresis 2013, 34 (11), 1493–1509. https://doi.org/10.1002/elps.201300021.
3. Fritsch, R. J.; Krause, I. Electrophoresis. Encyclopedia of Food Sciences and Nutrition (Second Edition) 2003, 2055–2062. https://doi.org/10.1016/B0-12-227055-X/01409-7.
4. Walter, N. Ion Mobility. https://websites.umich.edu/~chem260/winter00/lecnotes/lecture35.pdf (accessed 2023-04-16).
5. Garcia-Schwarz, G.; Rogacs, A.; Bahga, S. S.; Santiago, J. G. On-Chip Isotachophoresis for Separation of Ions and Purification of Nucleic Acids. JoVE 2012, No. 61, 3890. https://doi.org/10.3791/3890.
6. Rogacs, A.; Marshall, L. A.; Santiago, J. G. Purification of Nucleic Acids Using Isotachophoresis. Journal of Chromatography A 2014, 1335, 105–120. https://doi.org/10.1016/j.chroma.2013.12.027.
