User:Anthony Salvagno/Notebook/Research/2009/11/06/Oligo Designing
I want to troubleshoot the ligation. The first method of doing so is to check to make sure BstXI is cutting the PCRed plasmid. So I need to design an oligo that will serve as an adapter between two anchor segments. I sent Koch a mock one yesterday, realized it was wrong and didn't have time to redo it. So now I am doing everything. Also, I will look into increasing the PCR fragment size. This will allow me to actually visualize digestion on a gel. For this I will need to design primers.
Concept for BstXI adapter
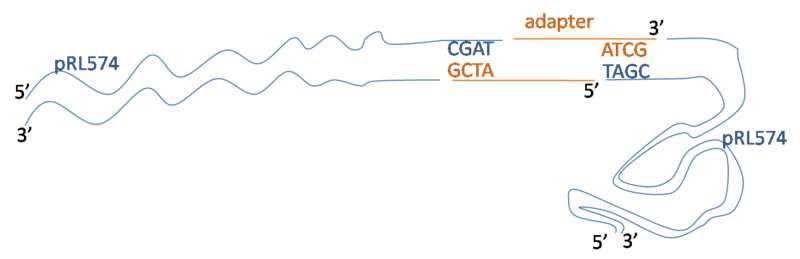
This is what I want. On the left is the pRL574 fragment. In order to check for digestion, I will need to ligate two of these fragments together. So in between two pRL574 fragments I have some adapter. It has two 3' overhangs complimentary to the BstXI overhang.
Design of BstXI adapter
I'm going to modify the top adapter with the BstXI overhang so that I can ligate the two together:
Name: Top Adapter -- BstXI / SapI Scale: 200 nmol Sequence 5' to 3': 5’-Phosphate- GCTGTCTGAATTCTAATGTAGTATAGTAATCCGCTCATCG Modification: 5'-phosphate Purification: OPC (Unless you think PAGE would significantly improve purity)
Now I want a complimentary bottom sequence with a 3' BstXI overhang, but I don't want to compliment the BstXI overhang on the top strand:
Name: Top Adapter -- BstXI / SapI Scale: 200 nmol Sequence 5' to 3': 5’-Phosphate- GAGCGGATTACTATACTACATTAGAATTCAGACAGCATCG Modification: 5'-phosphate Purification: OPC (Unless you think PAGE would significantly improve purity)
The easiest method would be to have these two oligos, but it could be costly. I am thinking about making half the oligo self complimentary so that I would just need to purchase one oligo, but then I could get lots of hairpins and cap my PCR fragment. The two oligo method is better. (Steve Koch 15:32, 6 November 2009 (EST): I agree the two oligo method is better, and it's really not that costly, since it's not biotinylated. I just thought of something: couldn't you do this with a biotin/nick, and actually produce unzippable pRL574? it's not versatile for unzipping downstream stuff, but it is a method for creating unzippable DNA as a start.)
mFold of BstXI adapter

Inputs:
- all default
If Koch approves, then this is what I will have. (Steve Koch 15:39, 6 November 2009 (EST): It looks pretty good to me. And I guess since you're ordering an internal biotin, email to Victor is the best way (the above format is usually how I send him email: plain text...I'll post an example email.) /email snippet from AlphaDNA
Steve Koch 15:43, 6 November 2009 (EST): Actually, found a better email. You can use the excel sheet with an "X" /AlphaDNA explanation of how to order
Concept of new primer design
Design of Primers
To figure out proper primer design I am referring to the God-written Molecular Cloning lab manual. Here are some things I should look for:
- 40-60% G-C content
- ~18-25 nucleotides long
- primer pair should not differ by more than 3bp
- no complimentarity between primer pairs
- should end in G or C, but should not end in GC or CG
- melting temps of primer pair should not differ by more than 5C and the Tm of the amplified product should not differ from the primer pair by more than 10C
Since this will be a brand new PCR reaction, I will have to troubleshoot all over again so I can choose whichever forward primer I want. F853D melts at 68.2C and F834D melts at 67.2C. There are some sweet PCR tools out there and I am trying this one:
Primer3
I have one problem so far. It says that F853D is not a suitable primer cause it has too high of a melting temp. It melts at 68C according to mFold, so maybe that's right. But the primer that the program told me to use melts at 68C (mfold). That isn't any different. Also the Tm that Primer3 calculates are different than mfolds. For F853 it got 63.97C and for the given one Tm was 59.72C. So I'm going to assume that F853D is a good primer (cause it works in PCR) and that its primers also work.
Here is my primer:
- 5' - ACTGGTTTTCCGCCATTTC named R2342
I used these parameters (if I don't mention it below then I left the field blank or at its default value):
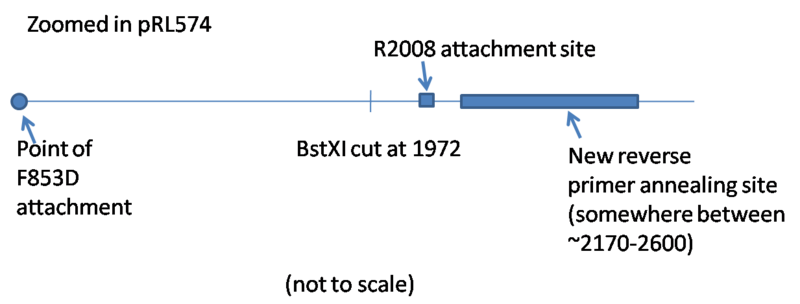
- Targets 1969, 4 - this makes sure the sequence of the BstXI overhang is in the product
- Product Min - 1300
- Product Max - 1700
- Product Opt - 1500
- Primer GC% Min 40, Max 60
mFold analysis

- Tm = 73.7C for duplexing
- Tm = 43C for self hybridization
Even though there is some self hybridization, it actually looks alright, plus Tm is below all PCR temps. So I will get this.