User:Omar Choudary/Notebook/Omar Choudary CHEM-571 AU-2011/2012/2012/04/04
 Chem-571 Chem-571
|
|
|
Objective
Description and Data
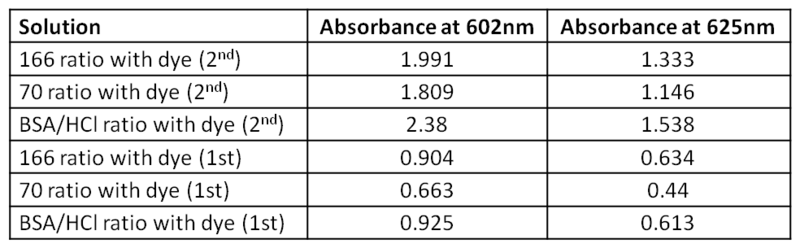
1. Determining concentration of the dye in solution. The dye in solution was prepared on the 2/21 using: mass = 1 mg of dye in 5 ml of water + 80µL of DMSO = Total volume (V) = 0.0058L MW of dye = 573.51 g/mol So, n = 10^-3/573.51 = 1.74* 10^-6 moles And, concentration of dye in solution (c) = (1.74* 10^-6)/0.0058 = 3*10^-4 M 2. Determination of the molar absorptivity of the dye using Beer's law. Use the Absorbance at 602nm (which is the absorption value given by the dye manufacturer); at this wavelength, fluctuations should be the lowest. Molar absorptivity (ε, L/mol/cm) were determined using Beer's law: A = ε*l*c With A = absorbance at 602nm l = width of the cuvette (1cm) c = dye concentration (3*10^-4 M) The molar absorptivity of the dye at 602nm = 3419 L.mol^-1.cm^-1 (Average of the three different ε values) Determination of the molar absorptivity of the dye (at 625nm) using a known-concentration solution. Molar absorptivity (ε, L/mol/cm) were determined using Beer's law: A = ε*l*c With A = absorbance at 625nm l = width of the cuvette (1cm) c = 3*10^-4 M The molar absorptivity of our dye at 625nm is equal to = 2204 L.mol^-1.cm^-1 ( Average of the three different ε values) Determination of the dye concentration in 166, 70 and BSA/HCl solutions (using 3/27 UV spectrum). In this table are corrected value for Absorbance (Abs with dye - Abs without dye) According to the data, we can calculate the dye concentration using Beer's law. We know that: ε at 625nm = 2204 L.mol^-1.cm^-1 ε at 602nm = 3419 L.mol^-1.cm^-1 Notes
Using the UV-VIS of a known dye standard, the molar absorptivity of the dye was determined using Beers Law The molar absorptivity value calculated from the UV spectra of the dye was too low, so used the value obtained in literature Beer’s Law and the calculated molar absorptivity were used to determine the concentration of dye in all of the dye reactions To account for the presence of gold nanoparticles and BSA solutions, corrected values for absorbance were used. The corrected values were acquired by subtracting the absorbance of the control solutions (166, 70, and BSA/HCl without dye) from the absorbance values obtained from the spectra for each trial Used the initial concentrations of BSA and assumed that no BSA had been lost during the experiment
Hypothetically 50% of Lys are bound with a dye molecule in 2nd trial | |