Protein blot (Western)
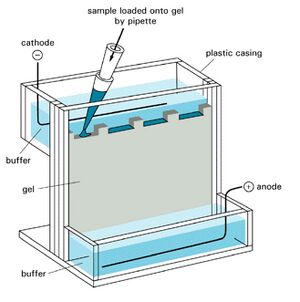
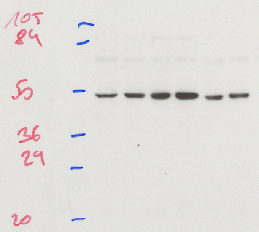
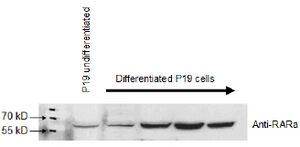
A protein blot, also known as the immunoblot or Western blot, is a method of semiquantitative determination of protein expression. Crude cell lysates are loaded into a polyacrylamide gel containing a denaturing agent which give all the proteins a net negative charge. A current passing through the gel will then propel the proteins through the gel, with the largest proteins travelling the slowest. This results in the proteins being roughly separated by their mass. The proteins are then transferred from the gel onto a membrane (often nitrocellulose or PVDF), which is then incubated with an antibody directed against the protein of interest. By using a detector conjugated to an antibody one can then specifically detect the protein of interest.
Standard western blot procedure
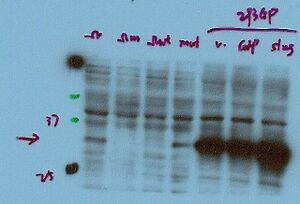
Prior to running the western blotting experiment, consult with the vendor in order to determine if they have optimized the titration for the antibody you just purchased. Most vendors should offer some guideline for how to use the antibody. Value your time and effort first and foremost. If the experiment comes out strange, make the call or email the vendor for assistance. Your time is the most valuable element to the procedure!
Mouse monoclonal western blot
- Load at least 50 ug of whole cell lysate or 20 ug nuclear extract prepared from fresh buffers (30 µg of protein ~ 25,000 cells). Resolve proteins on the gel voltage gradient.
- Transfer proteins to a nitrocellulose or PVDF membrane and block the membrane in 10 cm square dish with fresh 5% milk TTBS 2 hr room temperature, or overnight at 4C.
- Perform 3-5 shake rinses with cold TTBS (until no residual milk is observed).
- Incubate the primary antibody 1:200 in fresh 3% milk TTBS for 90 minutes room temperature.
- Perform 3 shake rinses, followed by 4X for 5 minutes washes with ambient or cold TTBS.
- Incubate the the secondary antibody 1:1000 anti-mouse in fresh 1% milk TTBS for 60 minutes at room temperature.
- Perform 3 shake rinses, followed by 4X for 5 minutes washes with ambient or cold TTBS.
- Perform ECL using a suitable detection reagent. Wick away all residual chemiluminescent liquid by dabbing membrane edges on paper towel.
- Prepare the membranes for ECL by covering with either saran wrap, or a transparency page. There should be no running of ECL liquid.
- Optimize protein signal with multiple exposure times.
Goat polyclonal western blot
- Load at least 50 ug of whole cell lysate or 20 ug nuclear extract prepared from fresh buffers (30 µg of protein ~ 25,000 cells). Resolve proteins on the gel voltage gradient.
- Transfer proteins to a nitrocellulose or PVDF membrane and block the membrane in 10 cm square dish with fresh 5% milk TTBS 2 hr room temperature, or overnight at 4C.
- Perform 3-5 shake rinses (until very little residual milk washes off the membrane).
- Incubate membrane with the primary antibody 1:200 in fresh 5% Milk TTBS for 90 minutes room temperature.
- Perform 3 shake rinses, followed by 4X 5 minute wash with ambient or cold TTBS.
- Incubate the the secondary antibody 1:5000 anti-goat in fresh 5% Milk TTBS for 60 minutes at room temperature.
- Perform shake rinses, until no residual milk is observed, followed by 4X 5 minute wash with ambient or cold TTBS.
- Perform ECL using a suitable detection reagent. Wick away all residual chemiluminescent liquid by dabbing membrane edges on paper towel.
- Prepare the membranes for ECL by covering with either saran wrap, or a transparency page. There should be no running of ECL liquid.
- Optimize protein signal with multiple exposure times.
Rabbit polyclonal western blot
- Load at least 50 ug of whole cell lysate or 20 ug nuclear extract prepared from fresh buffers (30 µg of protein ~ 25,000 cells). Resolve proteins on the gel voltage gradient.
- Transfer proteins to a nitrocellulose or PVDF membrane and block in 10 cm square dish with fresh 5% milk TTBS 2 hr, r.t. or overnight at 4C.
- Perform 3 shake rinses (until very little residual milk washes off the membrane).
- Incubate the primary antibody 1:200 in fresh 3% milk TTBS for 90 minutes room temperature.
- Perform 3 shake rinses, followed by 4X 5 minute wash with ambient or cold TTBS.
- Incubate the the secondary antibody 1:4000 anti-rabbit in fresh 1% Milk TTBS for 60 minutes at room temperature.
- Perform 3 shake rinses, followed by 4X 5 minute wash with ambient or cold TTBS.
- Perform ECL using a suitable detection reagent. Wick away all residual chemiluminescent liquid by dabbing membrane edges on paper towel.
- Prepare the membranes for ECL by covering with either saran wrap, or a transparency page. There should be no running of liquid.
- Optimize protein signal with multiple exposure times.
Phosphorylation state specific western blot
Adjusting certain incubation conditions can improve the detection signal for the phospho-specific antibody. When serine/threonine phosphatase inhibitor Sodium Fluoride (NaF), and tyrosine phosphatase inhibitor Sodium Orthovanadate (Na3VO4) are included in the blocking and incubation buffers, phospho-specific signals can noticeably improve. Including 50 mM NaF and 5 mM Na3VO4 in the blocking and incubation buffers can improve the signal. Using 5% milk diluent for primary and secondary incubations will reduce the nonspecific banding and background.
- Load 50 ug of whole cell lysate/tissue extract OR 20 ug of nuclear extract prepared from fresh buffers. Resolve proteins on the gel voltage gradient.
- Transfer proteins to a nitrocellulose or PVDF membrane and block in fresh 5% milk TTBS, 50 mM NaF/5 mM Na3VO4 for 2 hr, r.t. or overnight at 4C. Perform 3 shake rinses and drain until no residual milk appears to stream off the wash buffer.
- Incubate the primary antibody 1:200-20000 in fresh 5% milk TTBS, 50 mM NaF/5 mM Na3VO4 for 120 minutes room temperature. Perform 3 shake rinses followed by wash with cold TTBS 4X for 5 minutes each wash.
- Incubate the the secondary antibody 1:2000 anti-mouse, 1:5000 anti-rabbit, 1:5000 anti-goat in fresh 5% milk TTBS, 50 mM NaF/5 mM Na3VO4 for 60 minutes at room temperature. Perform 3 shake rinses followed by wash with cold TTBS 4X for 5 minutes each wash.
- Perform ECL using a suitable chemiluminescent reagent
- Optimize protein signal with multiple exposure times.
Lambda Phosphatase (Dephosphorylation)
Lambda Phosphatase Lysate Treatment
Lambda Phosphatase (Lambda PP) is a Mn2+-dependent protein phosphatase with activity towards phosphorylated serine, threonine and tyrosine residues. The enzyme is inhibited by vanadate ions and can be heat inactivated at 65° C for 1 hour in the presence of 50 mM EDTA
- To a micro-centrifuge tube add 80 µg of lysate. Amount maybe scaled up or down as needed.
- Add 6 microliters of lambda-PPase Buffer. Final concentration: 50 mM Tris, pH 7.5, 0.1 mM Na2EGTA, 5 mM dithiothreitol, 2 mM MnCl2.
- Add 400 units of Lambda-Phosphatase.
- Incubate at 30˚C for 20 minutes.
- The protein sample/lysate is now ready for analysis or further preparation depending on application
- Sharma SK and Carew TJ. Inclusion of phosphatase inhibitors during Western blotting enhances signal detection with phospho-specific antibodies. Anal Biochem. 2002 Aug 1;307(1):187-9. DOI:10.1016/s0003-2697(02)00008-8 |
Membrane Treatment
Treats ~30 lanes or 2-3 (6x12 cm) membranes. Mark treated and untreated membranes.
- Block membranes in 3%BSA, 1X TBS, 0.1% Triton X-100.
- Add 15 ul of Lambda-PPase (Santa Cruz Biotechnology Inc. sc-200312) in 15 ml of blocking buffer + 2mM DTT, 2mM MnCl2.
- Incubate submerged membrane @ room temp 4hr, or 4C ON.
- Wash 2x 1X PBST.
- Wash 2x dH2O.
- 1ary incubation / 2ary incubation / wash / ECL.
Heat dependent antigens
Certain antigens (caveolin-1) have been reported to be heat sensitive in SDS-PAGE analysis. Antigen sensitivity may be improved by adjusting lysis heating parameters from
95C for 3-5 minutes
TO
70C for 10 minutes
Transfer tips for high molecular weight proteins
Standard protein transfer
1) Perform electrophoresis on 8-15% acrylamide SDS-PAGE gel, according to standard procedures.
2) Transfer proteins onto a nitrocellulose membrane, overnight (approximately 18 hours) at 0.1 Amp.
3) Running Buffer: 50 mM Tris: 6.06 g Tris Base, 380 mM Glycine: 28.5 g Glycine, 1.6 mM EDTA: 0.67 g EDTA (tetra Na is the most soluble), 0.1% SDS: 1.0 g SDS
4) Transfer buffer: 50 mM Tris, 380 mM glycine, 0.1% (w/v) SDS, 10% (v/v) methanol. Final pH: 8.3.
Adjustments to consider for high molecular weight proteins
- 6% acrylamide gel is preferable with high molecular weight proteins.
- Use 15-20% methanol in transfer buffer. Methanol strips the SDS off of the proteins, which changes the charge of the proteins. Methanol also causes the gel to shrink.
- Do not let the gel sit in transfer buffer for prolonged periods prior to transfer.
- Transfer for 16 hours at 30 Volts, then 1 hour at 100 Volts. Do not exceed 220 mAmps.
- O.45 um PVDF or nitrocellulose may be effective.
Transfer tips for low molecular weight proteins
Tricine Gels are ideal for protein separation from 2.5-50 kDa.
- Tricine Gel
- Gradient 10-20%
- 0.1 or 0.05um PVDF or 2 pieces of 0.2 micron PVDF
- lower transfer time relative to standard transfer time
Tricine Gel
Tricine ions migrate ahead of the smallest proteins. This improves the resolution of low molecular weight proteins. Tricine gels display good transfer efficiency, in a lower pH.
Reducing vs Non-Reducing Conditions
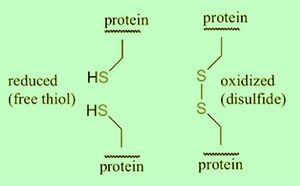
Disulfide (sulfur-sulfur) bridging can occur across two cysteine residues near eachother, and influence protein conformation in three-dimensional protein structure. Interconversion between thiols and disulfide groups is a redox reaction: thiol an reduced state, and disulfide an oxidized state.
Reducing conditions incorporates beta-mercaptoethanol (2-ME), Tris(2-carboxyethyl)phosphine hydrochloride (TCEP•HCl), dithiothreitol (DTT) to reduce (free thiol (-SH)) disulfide bridges in proteins. Removal of salt bridges improves protein denaturation (linearization) for resolving in SDS-PAGE. Reducing condition SDS-PAGE is a common and reliable strategy for protein detection by Western blotting.
Antibodies tend to favor reducing conditions. However there are instances where epitope(s) are sensitive to reducing forms vs non-reducing conditions. Non-reducing conditions tend to cause a smearing effect as protein rate migration is altered, whereas reducing conditions normalizes, and greatly improves protein resolution.
Beta-mercaptoethanol 2-5% (vol:vol) (fresh addition to 2x loading buffer)
DTT 50mM final concentration (fresh addition to loading buffer) ; can also be used in lysis buffers at low concentrations (1-10 mM) to stabilize free sulfhydryl group containing proteins.
Tris(2-carboxyethyl)phosphine hydrochloride (TCEP•HCl) 50mM final concentration (fresh addition to loading buffer).
Protein Deglcyosylation

Glycosylation of proteins and lipids is a post-translational modification important for numerous physiological processes; protein folding, ligand binding, cell surface contour, and influencing cell–cell and cell–matrix interactions.
In order to determine the presence and/or significance of protein glycosylation, the carbohydrate chains may be removed by catalysis.
N-Glycosidase F (PNGase F) is a ~36 kDa amidase that cleaves at the innermost N-acetylglucosamine (GlcNAc) and asparagine residues of high mannose, hybrid, and complex oligosaccharides from N-linked glycoproteins.
- PNGase F
- Endo-O-Glycosidase
- a-2(3,6,8,9)-Neuraminidase (Sialidase A)
- ß(1,4)-Galactosidase
- ß-N-Acetylglycosaminidase
(1) Tarentino, A.L. and Plummer, T.H., Jr. (1994) Methods in Enzymology, 230: 44-47. PMID: 8139511
Deglycosylation kits
Enzymes and reagents combined into convenient to use kits exist in order to remove N-linked and O-linked carbohydrates from glycoproteins, and cleave complex core O-linked carbohydrates (including polylactosamine). Deglycosylation can simplify SDS-PAGE by decreasing the potential broad size variation associated with glycoproteins.
New England Biolabs offers a convenient kit Cat#P0704S.
Sigma Aldrich offers the PNGase F enzyme Cat#P7367.
Troubleshooting
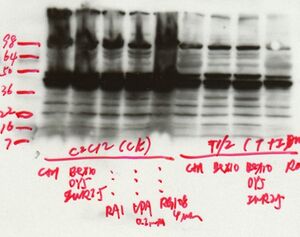
Blocking: To block nonspecific sites on the membrane, incubate the membrane with blocking buffer (5% milk TTBS) prior to primary antibody incubation. 2 hour room temperature blocking is sufficient for the membrane to absorb proteins and minimize noise.
Primary antibody: Use a diluent containing a blocking protein (5% milk TTBS or 1%milk/1%BSA TTBS) to dilute the primary antibody.
Secondary antibody: Use a diluent containing a blocking protein (5% milk TTBS or 1%milk/1%BSA TTBS) to dilute the primary antibody. Performing a "Secondary control" control blot where the primary antibody is purposely omitted is useful for identifying whether nonspecific signals are due to the secondary antibody conjugate or the primary antibody.
Washes: All wash steps are critical for reducing general background signal and nonspecific binding to discrete bands. If high background is a problem, the number, length and composition of the washes can all be increased.
General: Handle membranes carefully with forceps to minimize problems with nonspecific signals. Wear gloves for all steps to prevent hand contact with film, membranes or detection reagents.
PVDF versus Nitrocellulose
The western blotting procedures are the same for PVDF or nitrocellulose, however the handling of these membranes are different prior to- and during- transfer of proteins from the SDS-PAGE gel to the membrane.
Nitrocellulose
Nitrocellulose exhibits the highest sensitivity with very low backgrounds in all transfers, especially in protein blotting. Unlike PVDF, nitrocellulose wets out naturally, does not require methanol, and will not turn hydrophobic during the transfer process. Nitrocellulose is very easily blocked and does not need the many blocking steps required with PVDF. Protocols for Western Blotting with PVDF and Nitrocellulose are the same with a few exceptions.
- Nitrocellulose membranes are hydrophilic and can undergo hydration by aqueous buffers.
PVDF
Polyvinylidene Fluoride is hydrophobic and should be prewet in methanol before it is used.
- Wet the membrane in 100% methanol for 15 seconds. The membrane should uniformly change from opaque to semi-transparent.
- Place the semi-transparent membrane into ultrapure water and soak for 5 minutes.
- Place the membrane into transfer buffer and soak for at least 5 minutes.
- Transfer proteins to the smooth/reflective side that has more shine
Another change to note is that the SDS tolerances are not equivalent for PVDF and Nitrocellulose. The binding of protein to PVDF is much more sensitive to SDS levels. Too much SDS can inhibit the protein's ability to bind to the PVDF and can, in fact, help proteins already bound to the membrane to slip off. SDS levels should never exceed 0.05% for PVDF.
Ponceau S of PVDF
Ponceau S is a negatively charged, red colored stain that binds positively charged amino groups and non-polar regions of proteins at low sensitivity (>1ug protein threshold of detection).
- PVDF membrane is polar/hydrophobic. Aqueous buffers(transfer buffer, dH2O. etc) do not absorb into the membrane.
- //Pre-Transfer// Activate your PVDF: Prior to transferring protein from gel to PVDF, rinse/pre-wet/activate (15-30 seconds) the PVDF membrane in methanol (or other 100% alcohol (ethanol or isopropanol)) in order to hydrate/activate the membrane, and ensure efficient transfer/protein binding. Membane turns from opaque to semi-transparent.
- //Post-Transfer// Enhance your PVDF to receive aqueous Ponceau S; Prior to Ponceau S staining, rinse/prewet/activate (15-30 seconds) the PVDF membrane in methanol, post-transfer, immersing in methanol before a wash with water or PBS and proceed with Ponceau S staining.
Ponceau S Staining Solution
Incubate membrane in Ponceau S Staining Solution 1-10 minutes room temperature. Rinse membrane in ddH2O to achieve reddish-pink protein bands to desirable effect (1-5 min).
- (0.1%(w/v) Ponceau S in 5%(v/v) acetic acid)
1g Ponceau S
50ml acetic acid
Make up to 1L with ddH2O
Store at 4°C. Do not freeze. Alternative recipe for stain solution 0.2%(w/v) Ponceau S in 3%(v/v) acetic acid.
Membrane Stripping
Griffin:Membrane_Stripping_and_Reprobing
Stability of reagents
- Ammonium persulphate/persulfate (APS) is a very reactive compound (used for radical generation in the polymerization process). It is prone to degradation and loss of activity which will increase your polymerisation times. Make small aliquots, keep in the cold. [1]
- TEMED, the catalyst of polymerisation, is also prone to oxidation especially if mixed with water which it attracts if the container is left open. Seal quickly, keep in cold place. [2] [3]
Toxicity of reagents
- acrylamide/bis-acrylmide is a neurotoxin. Handle with precaution. [4]
- TEMED is very corrosive. Most likely exposure in the lab due to inhalation. Reseal the container as quickly as possible. [5]