"Experiments & Results"
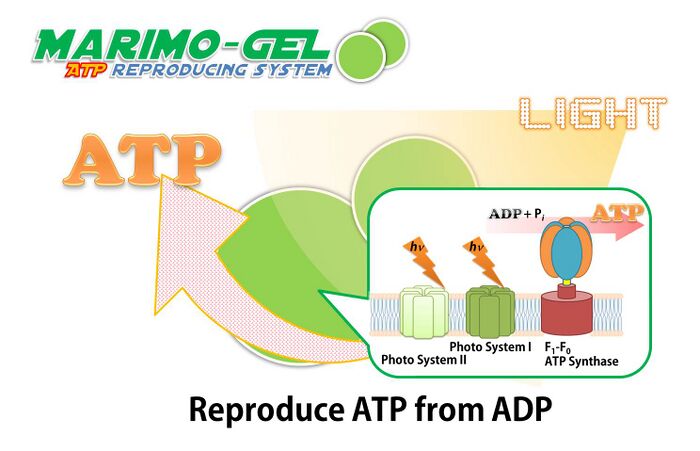
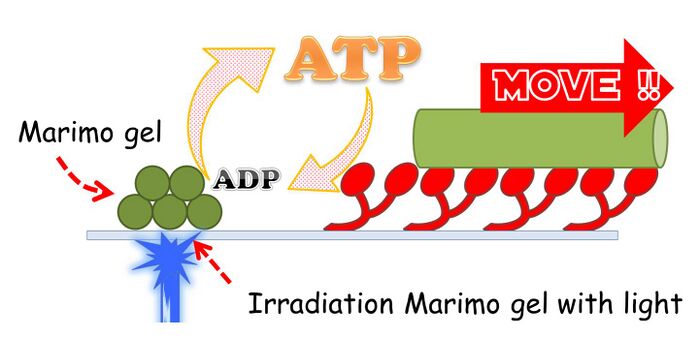
Our Marimo gel can produce ATP from ADP by utilizing the energy from light, because Marimo gel include ATP synthase and photosystem. MARIMO gel was synthesized from alginate and thylakoid membrane of spinach.
Schematic image of Marimo-gel reproducing ATP from ADP.
All purification procedures were performed at 4 oC. Thylakoid membranes were prepared from spinach leaves by the modified method of Yu et al. The spinach leaves (~160 g) were washed with deionized water and homogenized in 500 mL of homogenization solution (0.3 M sucrose, 20 mM NaCl, 5 mM MgCl2, 50 mM Tris-HCl, pH was adjusted to 7.6) for 40 sec using an AM-10 homogenizer (Nihon Seiki Seisakusho, Japan). The homogenate was filtered through four-time folded gauze. The flow through was suspended in 400 mL of a high ionic strength buffer (10 mM Hepes-KOH, 150 mM NaCl, pH8). The suspension was centrifuged at 10,000g for 20 min and the precipitate was resuspended in 15 mL of homogenization solution including 5% DMSO and flash frozen in liquid nitrogen, and stored in liquid nitrogen.
Thylakoid membrane was purified from spinach leaves.
(Yu A. H. C; Hosono K. Biotechnol. Lett. 1991, 13, 411.)
Experiment
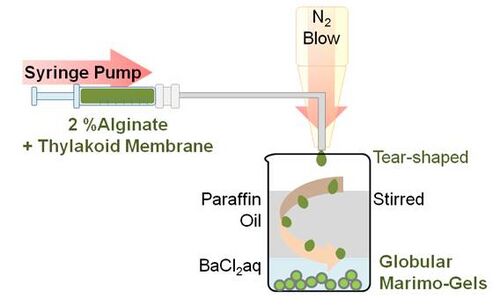
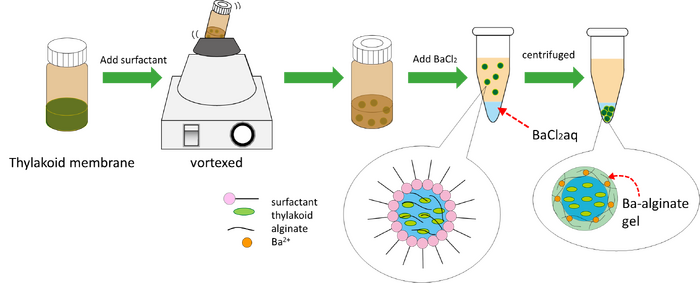
This procedure is based on the method described by Paul F. et al. and Zekorn T. et al. A 0.3 mL of suspension of thylakoid membrane containing 1.41 mg protein/mL and 2.7 mL of a 2%(w/v) sodium alginate solution (100 mM K3PO4,100 mM MgCl2, 100 mM NaCl, 500 mM PIPES, 200 mM ADP, pH was adjusted to 7.6) were placed in a 1 mL syringe. Using syringe pump (HA2000P, Harvard apparatus, US), the alginate solution including thylakoid membrane was slowly ejected from the needle and was blown by a nitrogen gas. The tear shaped green droplet firstly encountered mineral oil phase and transformed into globular shape. Then the droplet sunk into the second phase, which contains 50 mM BaCl2 and cross-linkage of alginate with barium occurred. Because leak was not observed even after few weeks from the encapsulation, the cross-linked alginate mesh seemed to be enough small to support thylakoid membranes.
Schematic image of synthesizing marimo gel by N2 blow.
(Paul F.; Vignais P. M. Enzyme Mcrob. Technol. 1980, 2, 281.)
(Zekron T.; Horcher A.; Siebers U.; Schnettler R.; Klock G.; Hering B.; Zimmermann U.; Bretzel R. G.; Federlin K. Acta Diabetol, 1992, 29, 99.)
Results
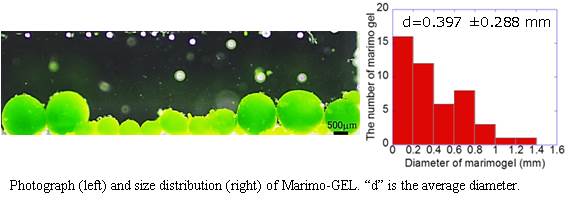
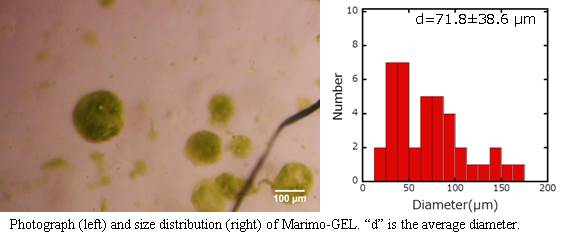
From the photograph and histogram, it is evident that Marimo-GEL of large size (>300 µm diameter) could be prepared, which not small enough by introducing into the flow cell.
Experiment
0.2 g/mL ER290 surfactant in paraffin oil 1000 µL was added on the mixture of 2 wt % alginate 100 µL and 2.1 mg/mL thylakoid membrane 100 µL in the brown bottle. The brown bottle was vortexed to form micell which was composed of the mixture of alginate and thylakoid membrane. The micell in the dispersion liquid was added on the 50 mM BaCl2 solution in a microcentrifuge tube. When the tube was centrifuged, Marimo-GEL was formed due to cross-link between alginate and Ba2+ ions.
Chemical structure of ER290
Schematic image of synthesizing Marimo-gel by using micro emulsion.
Results
From the photograph and histogram, it is evident that Marimo-GEL of very small size (<100 µm diameter) could be prepared, which small enough by introducing into the flow cell.
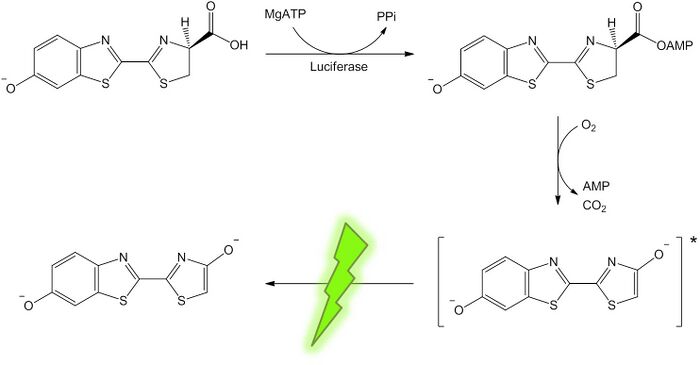
We examined the amount of regenerated ATP from Marimo gel by changing the irradiation time.Measurement of ATP concentration, Luciferin Luciferase reaction catalyzes the oxidation of luciferin in the pressence of ATP. Photons of light are released when oxyluciferin returns to ground state from exited state of oxyluciferin that is produced on the oxidation of luciferin. We measured the amount of photon by using photon counter to determine the concentration of ATP which was regenerated by Marimo-gel.
Schematic image of luciferin-luciferase reaction.
Experiment
1 mg of Luciferase (Sigma Aldrich) was dissolved in 10 mL of the HEPES buffer containing 15 mM MgSO4 at pH 7.8. The pH was adjusted using NaOH. 1 mg of luciferin was dissolved in 500 µL of the HEPES buffer. 20 µL of ATP at 5, 20, 50, 100, 200 mM was mixed with 20 µL of luciferin solution, 10 µL of luciferase solution, and 150 µL of the HEPES buffer. Photon production was measured by using a photon counter (Scientex) after 1 minute from mixing the ATP and luciferin-luciferase. The amount of different ATP concentration was determined. Standard solutions were made by using ATP(Wako).
Experiment
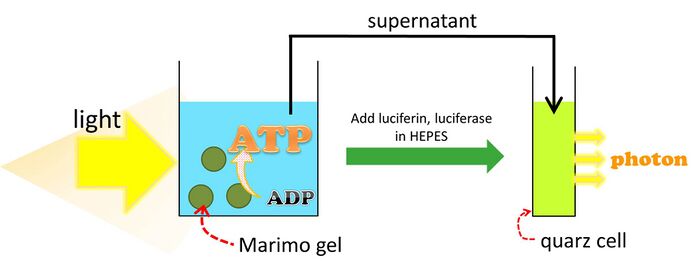
Marimo gel suspension was placed on the coverglass, and 455-485 nm light was irradiated for 4.5 h. The supernatant was mixed with luciferin-luciferase solution. The amount of light of any given onentration of ATP was determined.
Schematic image of determination the ATP concentration which produced by Marimo gel.
We examined the dependence of ATP concentration on the speed of microtubules driven by kinesin. In vitro Motility Assay was performed by changing concentrations of ATP. From this experiment, we determined the minimum concentration of regenerated ATP which require to move microtubules on kinesin by Marimo gel.
Experiment
Flow-cells were prepared by placing a coverglass (18 × 18 mm2) on a coverglass (24 × 60 mm2) equipped with a pair of double-sided tape to form a chamber of approximately 2 × 18 × 0.1 mm3 (W × L × H) in dimension. The flow cell was filled with casein solution (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, 0.5 mg mL-1 casein; pH adjusted to 6.8 using HCl). After incubating for 2 min with casein to mask the glass surface, 5 µL of 200 nM kinesin solution (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, 0.5 mg mL-1 casein, 1 mM DTT, 10 mM paclitaxel, 1% DMSO; pH 6.8) were introduced and incubated for 5 min to bind the kinesins to the casein coated surface. The flow cell was washed with 5 µL of motility buffer. 5 µL of MTs solution (250 nM in motility buffer) was then introduced and incubated for 2 min, followed by washing with 5 µL of motility buffer. Finally, the gliding motion of microtubules was initiated by applying 5 µL of the different concentration of ATP buffer(0.0005, 0.001, 0.0025, 0.005, 0.05, 0.5 mM ATP) and measured velocity of microtubule. This experiment was performed at room temperature.
Results
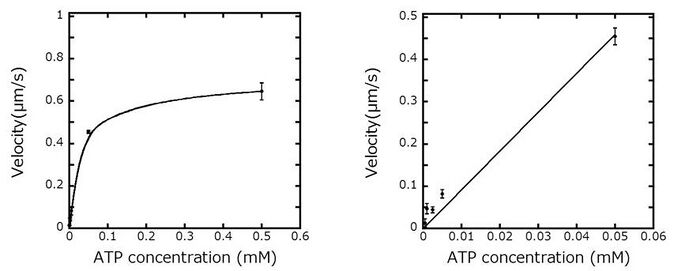
We prepared standard curve to calculate velocity of microtubules with ATP concentrations. We observed a linear relation between ATP concentration and microtubule velocity at low ATP concentration. This linear relation will be used for determining the concentration of ATP synthesized from Marimo-GEL by monitoring the velocity of microtubules.
Dependence of velocity of microtubules on the concentration of ATP (left) and initial part of the graph (right).
Experiment
BaCl2 solution in which Marimo-gels were soaked was substituted with motility buffer. 50 µL of Marimo-gels dispersion was mounted on the cover glass, and was exposed to 455-485 nm light for 4.5 h. 0.5 µL of 450 mg mL-1 D-glucose, 0.5 µL of 5000 U mL-1 glucose oxidase, and 0.5 µL of 5000 U mL-1 catalase were added to Marimo-gels dispersion. And then, this solution was centrifuged. Flow-cells were prepared by placing a coverglass (18 × 18 mm2) on a coverglass (24 × 60 mm2) equipped with a pair of double-sided tape to form a chamber of approximately 2 × 18 × 0.1 mm3 (W × L × H) in dimension. The flow cell was filled with casein solution (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, 0.5 mg mL-1 casein; pH adjusted to 6.8 using HCl). After incubating for 2 min with casein to mask the glass surface, 5 µL of 200 nM kinesin solution (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, 0.5 mg mL-1 casein, 1 mM DTT, 10 mM paclitaxel, 1% DMSO; pH 6.8) were introduced and incubated for 5 min to bind the kinesins to the casein coated surface. The flow cell was washed with 5 µL of motility buffer. 5 µL of MTs solution (250 nM in motility buffer) was then introduced and incubated for 2 min, followed by washing with 5 µL of motility buffer. Finally, the gliding motion of microtubules was initiated by applying 5 µL of the supernatant after centrifugation of Marimo-gels solution. This experiment was performed at room temperature.
Schematic image of in vitro Motility assay by using the ATP produced by Marimo-gel.
Results
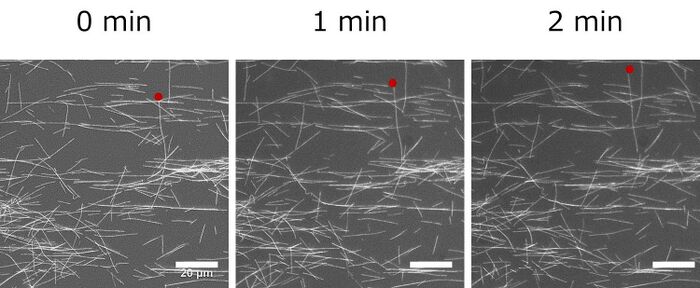
We observed movement of microtubules and measured velocity of microtubule. Average velocity of microtubules was 0.06 µm/s and we estimated ATP concentration was 0.0065 mM by using ATP standard curve.
Fluorescent image of microtubule driven by kinesin utilizing the ATP regenerated by Marimo gel.
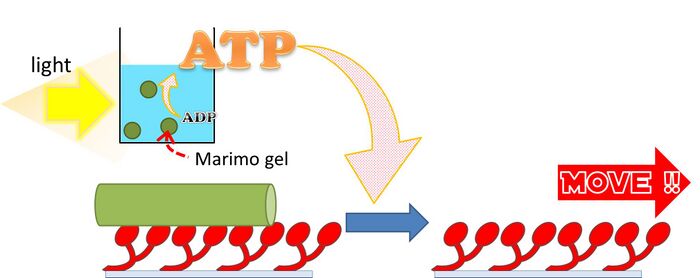
Mari-motor system
We established a new in vitro motility system which sustains for longer time without any supply of ATP from outside. We introduced Marimo-gel to in vitro motility system in order to regenerate ATP by using the F1FO ATP synthase and light enery. This new system is termed as Mari-motor system.
Schematic image of Mari-motor system where kinesin are driven by ATP produced by Marimo-gel.
Experiments
First, BaCl2 solution in which Marimo-gels were soaked was substituted with motility buffer. Next, flow-cells were prepared by placing a cover glass (18 × 18 mm2) on a cover glass (24 × 60 mm2) equipped with a pair of double-sided tape to form a chamber of approximately 2 × 18 × 0.1 mm3 (W × L × H) in dimension. The flow cell was filled with casein solution (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, 0.5 mg mL-1 casein; pH adjusted to 6.8 using HCl). After incubating for 2 min with casein to mask the glass surface, 5 µL of 200 nM kinesin solution (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, 0.5 mg mL-1 casein, 1 mM DTT, 10 mM paclitaxel,
1% DMSO; pH 6.8) were introduced and incubated for 5 min to bind the kinesins to the casein coated surface. The flow cell was washed with 5 µL of motility buffer. 5 µL of MTs solution (250 nM in motility buffer) was then introduced and incubated for 2 min, followed by washing with 5 µL of motility buffer. Finally, 5 µL of the Mario-gels dispersion (include 100 nM phenazine methosulfate) was applied. This experiment was performed at room temperature. For observing microtubules, samples were illuminated by green light (528-552 nm) with a 100W mercury lamp and visualized by epifluorescence microscopy using an oil-coupled Plan Apo 60×1.40 objective, where exposure time was maintained of 300 ms. Also for synthesizing ATP, Marimo-Gel was illuminated by blue light (455-485 nm), and exposure time was 5 s. We went time-lapse observation interval of 10 seconds.
Results
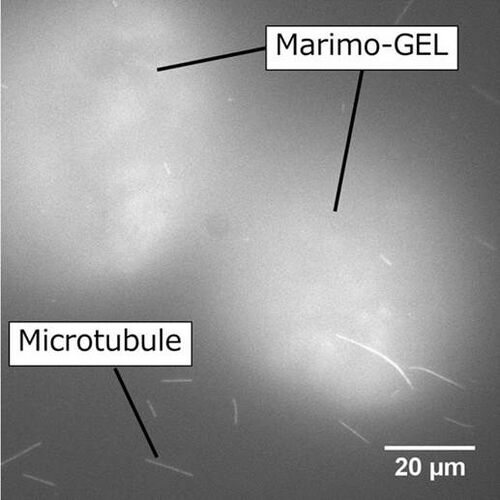
We could observe that Marimo-gel and microtubule were present in the same system. However, the motility of microtubule could not be observed. We are planning to increase the number of Marimo-gel in the system and the concentration of thylakoid membrane in Marimo-gel, and extend the time of radiation the gel with light.
Fluorescent image of Marimo-gel and microtubule on kinesin coated surface.