2D Mammalian Cell Culture - Min Zeng
Introduction
To maintain and grow mammalian cells in vitro is of great biological interest since mammalian cells are the dominant expression systems to produce therapeutic proteins[1] and are useful in the study of drug delivery and complex biological process, such as cell differentiation and tumor metastases.[2] The requirements and basic tools have not changed greatly over past decades.
Methods for Mammalian Cell Culture

Differentiated mammalian cell lines are mortal, which only survive limited cell passages, while undifferentiated cell lines, such as cancerous cells, are immortal, which can propagate indefinitely. Mammalian cells either grow on a support surface (anchorage-dependent cells) or in suspension culture (nonanchorage-dependent cells). For anchorage-dependent cells, they must attach to and spread on a surface to be viable. In macroscale culture, cells are attached on the glass surface of T-flask or roller bottles (Figure 1). Extracellular matrix can also be added to promote cell attachment. When mortal mammalian cells form a monolayer on a support surface and cells contact with each other, the cell division will be inhibited. Then proteolytic enzyme trypsin will be added to separate cell aggregate and continue cell passage.[4]
The traditional growth medium for mammalian cells includes carbon and energy source to provide nutrient, inorganic salts to maintain osmotic pressure and growth factors. Serum are most commonly used reagents to provide growth factors, which also contain amino acids, hormones, vitamins, lipids and minerals. The undefined compositions of serum sometimes complicate cultivation process thus defined media have been developed without serum for specific cell lines.[4]
Macroscale mammalian cell cultivation modes in research lab can be divided into non-stirred systems and stirred systems. Common non-stirred systems include T-flask and roller bottles and stirred system has spinners flask. Mammalian cells are usually cultured at 37 °C and supplemented with 5% CO2 to maintain the pH of media.[4]
Significance of Mammalian Cell Culture at Microscale
For some applications of mammalian cell culture, such as mesenchymal stem cell (MSC) therapies, [5] precisely controlled soluble and mechanical factors microenvironment are required for MSC differentiation. A controlled microenvironment is essential to extract biologically meaningful data from cell assays. Microfluidics devices, integrating cell culture, reactions and assays in a single platform, are especially attractive due to their potential for high-throughput screening and mimicking in vivo-like microenvironment by precise control over fluid flow and local gradient. [6] For anchorage-dependent cells, rigid substrates, such as plastic surfaces or glass, and soft substrates, such as polymers, are used in microfluidics devices.[7] This is called 2D mammalian cell culture. Here we discuss one basic mode of 2D mammalian cell culture: perfusion flow mode.
For highly perfused tissues types in vivo, such as kidney and liver, microfluidic perfusion mode may recapitulate physiological cellular microenvironment. By continuous supply and removal of media, the perfusion culture can create a definable microenvironment which would be otherwise impossible to achieve on a macroscale since the composition always changes in static culture and a continuous reactor in macroscale has large reagent volumes consumption. To design a robust microfluidic perfusion system, design, fabrication and micro-assays should be discussed in an integrated fashion.
Design of Microfluidic Devices
The design of microfluidic devices depends on the feature required by the application.[6]
Biomaterials
Firstly, biomaterials should be selected for microfluidic channels and adhesion substrate of cells. All materials should be non-toxic, and materials choice may be further narrowed by assay requirements such as electrical stimulation/recording, chemical sensing, optical transparency, etc. Materials for channels and substrate can be the same, such as glass substrates and glass channels, or different, such as glass substrate and poly(dimethylsiloxane) (PDMS) channels. PDMS is the most commonly used materials due to its low cost, high optical transparency for imaging and biocompatibility. Additionally, the flexibility of PDMS allows one to create valves and pumps in microfluidic systems. However, water vapor and organic solvent permeability limit the application of PDMS.[8]
Materials including silicon, silicon nitride, glass, and PDMS are most commonly utilized as cell substrates in 2D microfluidic perfusion culture. Cells attach to intermediate molecules, typically peptides or proteins, instead of cell substrates directly. This requires substrates to undergo surface modification to add intermediate molecules by physical adsorption or chemical bonding. Surface modification is a critical step to facilitate cell attachment in 2D microfluidic perfusion culture.
Cell Culture Chamber
The simplest 2D cell culture chamber design is growing cells as a 2D monolayer at the bottom surface of channel which have been used in some microfluidic perfusion devices (Figure 2).
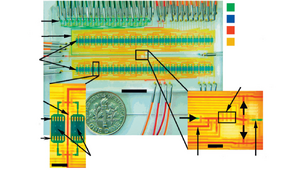
The effective culture volume (ECV) of culture chamber, characterized by large surface-area-to-volume ratio, measures the ability of cells to control their microenvironment. Laminar flow existing in culture chamber could be utilized for selective delivery of molecules. Several culture chambers connected by channels can be designed in a single microfluidic device to achieved parallelization. [10] Alternatively, some cell culture chambers are isolated from the bulk fluid to create a low shear stress environment.
Sterilization Techniques
To maintain prolonged periods of culturing, effective sterilization of microfluidic devices is critical. Autoclaving of microfluidic device and techniques used in experiment is one of the most efficient ways, while high temperature may cause damage to biomaterials or surface modification. Other sterilization methods can be used as substitutes, including incorporating sterilization chemicals during device fabrication, flushing with ethanol or exposing to UV light.
Operation of Microfluidic Devices
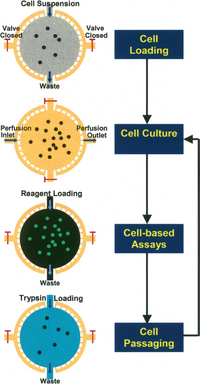
In perfusion culture, nutrients, chemicals and oxygen must be delivered to cells efficiently while wastes should be removed. For precise control of the microenvironment in 2D configurations, cell seeding and immobilization strategies can be used to ensure cell-cell and cell-matrix interactions (Figure 3).[6]
Cell Seeding
Cell seeding is the process of infusing external cell suspension into cell culture chamber, which is a dynamical process in microfluidic perfusion culture. The seeding cell density is critical due to its effect on the extent of cell-cell interactions and cellular behavior. The seeding flow rate should be low to decrease shear stress which could compromise cell viability. However, low flow rate can cause precipitate during seeding process and consequently non-uniform final cell density. In 2D microfluidic perfusion system, enough time should be allowed for sufficient cell attachment so that cells are not washed away by media perfusion.
Non-recirculating Culture vs. Recirculating Culture
After cells are immobilized, the perfusion starts. Two different modes of perfusion exist, non-recirculating culture and recirculating culture. Non-recirculating culture is a counterpart of continuous reactor in macroscale, where the fresh culture media is continuously injected into the system and go to waste directly.[11] [12]The local concentration of testing factor can remain constant during this testing process. Recirculating culture is a counterpart of batch reactor in macroscale, where a relatively large volume of culture media circulates in the system (Figure 4).[13]
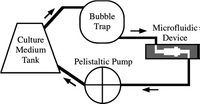
Operating Environment
The microfluidic device can be placed in 37 °C, 5% CO2 mammalian cell incubator, however, given this live imaging and other assays would become hard to achieve. this can be aided by placing the device on a 37 °C hot plate to maintain the temperature. In the non-recirculating mode, acidic metabolites will be washed away and thus CO2 is not necessary to maintain pH. In the recirculating mode, the addition of HEPES buffer can maintain culture media at a constant pH between 7.2 – 7.4 under atmospheric conditions. Minimizing light exposure to culture system is necessary when operating outside of incubator since reactive oxygen species can be generated by light.
Perfusion Flow Rate
In recirculating mode of microfluidics, no flow rate can cause cell death due to the accumulation of waste and lack of nutrients. At a slow flow rate, the proliferation can be limited. At an intermediate flow rate, cell density can be increased with the flow rate. At a higher flow rate, most cells can be displaced, and morphology of remaining cells thereafter changed.[13][14] Similar experiment results were observed in the non-recirculating mode. Slowest flow rates resulted in poor proliferation and higher flow rates resulted in nearly confluent culture on surface.[7][11]
Mass Transport
Optimizing the mass transport aims to adjust nutrients and oxygen levels according to different cell types and control local concentration of metabolites. Most simple methods involve changing flow rate and cell culture chamber height. By increasing flow rate and culture chamber height, the delivery of adequate nutrients and removal of toxic waste products can be more efficient.
Limitation of 2D Culture
The 2D cell culture system as it stands currently is not accurate enough for simulating in vivo cellular microenvironment since cell-cell and cell-matrix interaction mechanical forces are not fully recapitulated. Alternatively, the 3D cell culture microfluidics platform allows for regulating these interactions and are promising for mirroring the in vivo cellular microenvironment more closely.[15]
References
- ↑ Zhu, J. "Mammalian cell protein expression for biopharmaceutical production." Biotechnology advances 30.5 (2012): 1158-1170. http://dx.doi.org/10.1016/j.biotechadv.2011.08.022
- ↑ Du, G.; Fang, Q.; Toonder, J. "Microfluidics for cell-based high throughput screening platforms—A review." Analytica chimica acta 903 (2016): 36-50. http://dx.doi.org/10.1016/j.aca.2015.11.023
- ↑ https://commons.wikimedia.org/wiki/File:Cell_culture_flat_flasks-set.jpg
- ↑ 4.0 4.1 4.2 Kargi, F.; Shuler, M.L. Bioprocess engineering: basic concepts. Prentice-Hall PTR, 2017. ISBN-13: 978-0137062706. https://www.pearson.com/en-us/subject-catalog/p/bioprocess-engineering-basic-concepts/P200000000611/9780137459469
- ↑ Godara, P.; McFarland, C.D; Nordon, R.E. "Design of bioreactors for mesenchymal stem cell tissue engineering." Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology 83.4 (2008): 408-420. http://dx.doi.org/10.1002/jctb.1918
- ↑ 6.0 6.1 6.2 Kim, L.; Toh, L.C.; Voldman, J.; Yu, H. "A practical guide to microfluidic perfusion culture of adherent mammalian cells." Lab on a Chip 7.6 (2007): 681-694. http://dx.doi.org/10.1039/b704602b
- ↑ 7.0 7.1 7.2 Hung, P.J.; Lee P.J.; Sabounchi, P.; Lin, R.; Lee, L.P. "Continuous perfusion microfluidic cell culture array for high‐throughput cell‐based assays." Biotechnology and bioengineering 89.1 (2005): 1-8. http://dx.doi.org/10.1002/bit.20289
- ↑ Regehr, K.J.; Domenech, M.; Koepsel, J.T.; Carver, K.C.; Ellison-Zelski, S.J.; Murphy, W.L.; Schuler, L.A.; Alarid, E.T.; Beebe, D.J. "Biological implications of polydimethylsiloxane-based microfluidic cell culture." Lab on a Chip 9.15 (2009): 2132-2139. http://dx.doi.org/10.1039/b903043c
- ↑ Gomez-Sjoeberg, R.; Leyrat, A.A.; Pirone, D.M.; Chen, C.S.; Quake, S.R. "Versatile, fully automated, microfluidic cell culture system." Analytical chemistry 79.22 (2007): 8557-8563. https://dx.doi.org/10.1021/ac071311w
- ↑ Lee, P.J.; Hung, P.J.; Rao, V.M.; Lee, L.P. "Nanoliter scale microbioreactor array for quantitative cell biology." Biotechnology and bioengineering 94.1 (2006): 5-14. http://dx.doi.org/10.1002/bit.20745
- ↑ 11.0 11.1 Kim, L.; Vahey, M.D.; Lee, H.Y.; Voldman, J. "Microfluidic arrays for logarithmically perfused embryonic stem cell culture." Lab on a Chip 6.3 (2006): 394-406. http://dx.doi.org/10.1039/b511718f
- ↑ Chung, B.G.; Flanagan, L.A.; Rhee, S.W.; Schwartz, P.H.; Lee, A.P.; Monuki, E.S.; Jeon, N.W. "Human neural stem cell growth and differentiation in a gradient-generating microfluidic device." Lab on a Chip 5.4 (2005): 401-406. http://dx.doi.org/10.1039/b417651k
- ↑ 13.0 13.1 13.2 Leclerc, E.; Sakai, Y.; Fujii, T. "Cell culture in 3-dimensional microfluidic structure of PDMS (polydimethylsiloxane)." Biomedical microdevices 5.2 (2003): 109-114. https://doi.org/10.1023/A:1024583026925
- ↑ Gu, W.; Zhu, X.; Futai, N.; Takayama, S. "Computerized microfluidic cell culture using elastomeric channels and Braille displays." Proceedings of the National Academy of Sciences 101.45 (2004): 15861-15866.https://dx.doi.org/10.1073/pnas.0404353101
- ↑ Toh, Y.C.; Zhang, C.; Zhang, J.; Khong, Y.M.; Chang, S.; Samper, V.D.; Noort, D.V.; Hutmatcher, D.W.; Yu, H. "A novel 3D mammalian cell perfusion-culture system in microfluidic channels." Lab on a Chip 7.3 (2007): 302-309.http://dx.doi.org/10.1039/b614872g
