BISC209/S11: Streaking for Isolation
Protocol for Streaking for Isolation
Nutrient agar plates can be streaked using a three-phase or a four phase pattern (Figure A-1). YouTube Demo [1]
The handling of the plate can be accomplished in a number of ways, all of which attempt to minimize possible contamination by either keeping the lid over the plate or by keeping the plate upside down (Figure A-2).
Isolation streak technique: from broth, slant, or plate to plate.
1. Follow the protocol for BISC209/S11: Aseptic Transfer. If using a loop, sterilize it in a bunsen burner flame and allow the loop to cool for a few seconds. Not allowing any cap, lid, or plug to leave your hand, dip your loop in the donor broth culture or touch your loop to a pure colony of bacteria on a streak plate.- If using a broth and a loop, allow the film of liquid inside the loop to “break” in the tube so that only those cells adhering to the wet wire and not those in a film within the wire are transferred.
- If using a broth and a sterile cotton swab, remove the sterile cotton swab from its container; be sure not to touch the swab to anything. Insert the swab into the stock culture tube, roll the swab on the side of the container to remove excess liquid.
- If using a loop and either a colony from a plate or growth on the surface of a slant, touch the sterilized loop to the colony or growth to remove a small amount.
2. Using the swab or the loop, streak the first section of the plate using tight sweeping lines that stay within that section: (1/3 for a 3-phase pattern) or (1/4 for 4-phase) of the plate. It is fine to overlap your streaks in this section. (Fig A-3)
3. If you used a cotton swab to inoculate section 1, switch to the loop for the remaining steps.
4. Sterilize the loop and allow it to cool in the air for 15 seconds. Touch the loop to an unused edge of the agar surface to cool it completely before continuing.
5. Pull the loop through the previous streak (section 1) one or two times to re-inoculate the loop with cells. Now streak section 2 of the plate, avoiding section 1 after the first 1-2 streaks and trying not to overlap the streaks (Figure A-4).
6. Sterilize the loop and allow it to cool in the air for 15 seconds. Touch the loop to an unused edge of the agar surface to cool it completely before continuing.
7. Pull the loop through one edge of the streak in section 2 of the plate to obtain inoculum. Now streak the 3rd section of the plate.
8. Sterilize the loop and repeat 6 and 7 until you complete inoculating all sections of your plate. Incubate and look for isolated colonies that have grown from one cell (Figure A-5).

Figure A-1: Two patterns for labeling the bottom of a plate for Isolation Streak technique. The inoculating loop is sterilized between each section and inoculum is taken from the preceeding section to an uninoculated section of the plate.
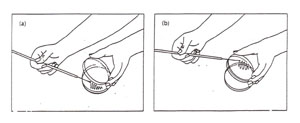
Figure A-2: Two options for aseptic transfer into a plate. (a) Streaking a plate while holding the lid ajar. Note that the lid shields the agar from airborne contamination: (b) streaking a plate while holding the bottom of the plate. Note that the agar surface faces downward, thereby minimizing contamination from the air.

Figure A-3: Pattern for isolation streaking section 1 of a plate.

Figure A-4: Illustration of isolation streak technique section 1 to section 2.

Figure A-5: An example of growth 24-72 hours after isolation streaking a plate to obtain isolated colonies.
Links to Labs
Lab 1
Lab 2
Lab 3
Lab 4
Lab 5
Lab 6
Lab 7
Lab 8
Lab 9
Lab 10
Lab11
Lab 12
