BME100 f2016:Group3 W8AM L1
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
|
OUR TEAM
LAB 1 WRITE-UPHealth Care IssueAlzheimer's Disease is an aggressive form of dementia in which a, "...progressive and irreversible cognitive decline" occurs (1). Alzheimer's Disease affects 5.2 million Americans alone and within the country, about $100 billion is spent on care for these patients (2). This disease is projected to grow as life expectancy continues to increase, thus a cure or a preventative measure is necessary to prevent millions of more Americans from developing this disease. While the cure for AD is still unknown, recent studies suggest that the pathology of the disease relates to the formation of the Aβ amyloid in the brain (3). Moreover, it is the solubility of the Aβ that distinguishes a healthy brain from an Alzheimer's affected brain. In fact, an Alzheimer's brain contains approximately three times as much soluble Aβ than the average brain. However, the amount of insoluble Aβ in the brain does not specifically correlate with how advanced the disease is or how many amyloid plaques are in the brain affecting the disease. As technology advances, decreasing the amount of soluble Aβ in the brain may be the solution to reversing the effects of the disease, but for now, Alzheimer victims just take medications to slow down the progression of the disease (3). Currently, the only "solutions" to Alzheimer's disease are medications that slow the degradation of the disease by slowing the process by which the disease destroys neurotransmitters. The following drugs are used to mask the effects of Alzheimer's disease: Donepezil Galantamine Memantine Rivastigmine Donepezil and Memantine These drugs work in two different ways. Donepezil, galantamine, and rivastigmine use cholinesterase inhibitors to slow down the process of the disease breaking down neurotransmitters. Memantine, a NMDA receptor antagonist, focuses on the regulation of the glutamate which is a crucial neurotransmitter in the brain that is responsible for memory. This drug administers calcium to the cells to help regulate cell signaling in the memory and learning portion of the brain. As the Alzheimer's disease takes over patients' bodies, the risk of the patients forgetting to take their medication increases. Therefore, a caretaker is typically necessary to monitor the intake of medication on a regular basis to increase the advantages of the drugs. Paying for these caretakers or even falling in the role of caring for a loved one proves to be highly expensive. Furthermore, in order to take care of loved ones in these early stages of the disease, family or friend caretakers "lose over $15,000 in annual income as a result of reducing or quitting work to meet the demands of caregiving" on average (2). The simple act of forgetting to take medication can be the start of this financial loss that effects many families every year. In attempt to minimize this issue, our group plans to create a implantable device that distributes the medication to Alzheimer's patients in a timely manner. The design of the device is similar to that of an insulin pump, however, it will include key features such as a heart rate monitor, blood pressure monitor, etc. that the patients' doctors will have access to in order to make sure the patient is safe. Furthermore, in case of a fall or a panic attack, the heart rate of the patient will rise and someone will be able to immediately respond to the scene and prevent any further damage. Overall, our device will eliminate the need for close monitoring or a caretaker in the beginning stages of the disease, thus saving money, hospital visits, and allowing both the life of the patient and their family the opportunity to live a more functional life.
Huang, Xudong, Robert D. Moir, Rudolph E. Tanzi, Ashley I. Bush, and Jack T. Rogers. (2004) Redox-Active Metals, Oxidative Stress, and Alzheimer's Disease Pathology.
Annals of the New York Academy of Sciences. Retrieved from http://onlinelibrary.wiley.com/doi/10.1196/annals.1306.012/full
2 Latest Alzheimer's Facts and Figures.(2016). Alzheimer's Association. Retrieved from http://www.alz.org/facts/ 3 McLean, C. A., Cherny, R. A., Fraser, F. W., Fuller, S. J., Smith, M. J., Konrad Vbeyreuther, Bush, A. I. and Masters, C. L. (1999), Soluble pool of Aβ amyloid as a determinant of severity of
neurodegeneration in Alzheimer's disease. Ann Neurol., 46: 860–866. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/1531-8249(199912)46:6%3C860::AID-ANA8%3E3.0.CO;2-M/full
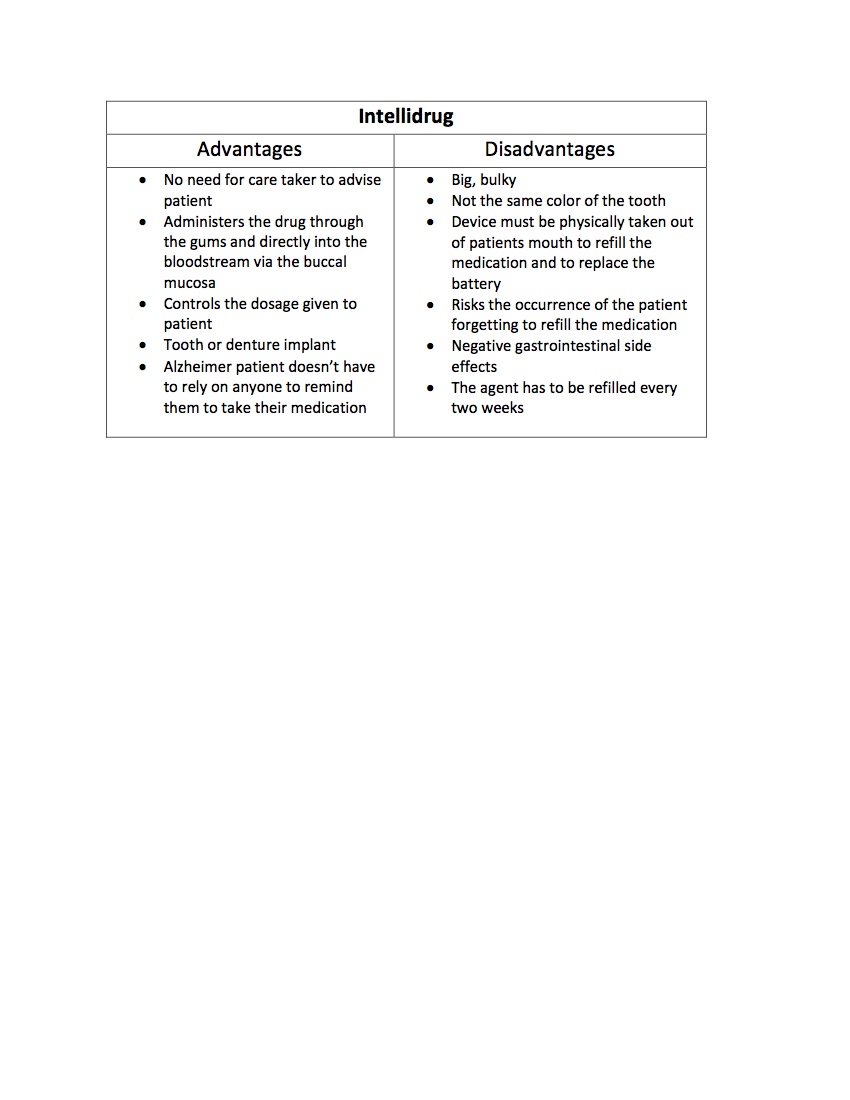
CompetitorsOur main competitor is an older device called Intellidrug which administered a Alzheimer-fighting drug to patients through an implantable device.
Citations: A.E., Moscicka, Czarnecka, K., & Ciach, T. (2007). IntelliDrug Implant for Medicine Deliveryin Alzheimer’s Disease Treatment. Retrieved August 31, 2016, from http://onlinelibrary.wiley.com/doi/10.1002/masy.200750720/epdf Giannola, L. I., Padern, C., De Caro, V., Florena, A. M., Wolff, A., & Campisi, G. (n.d.). New Prospectives in the Delivery of Galantamine for Elderly Patients Using the IntelliDrug Intraoral Device: In Vivo Animal Studies. Retrieved August 31, 2016, from http://www.eurekaselect.com/70972/article Hanlon, M. (2007, February 1). The Intellidrug tooth implant. Retrieved September 03, 2016, from http://newatlas.com/go/6778/ Moscicka, A. E., Czarnecka, K., & Ciach, T. (n.d.). IntelliDrug implant for medicine delivery in Alzheimer’s disease treatment. Retrieved August 31, 2016, from http://www.ifpan.edu.pl/paj/biomat/abstracts/pdf/a024_moscicka.pdf
Customer ValidationPatient Payer Physician Provider Purchaser
Sources: Accera, Inc. | A new hope in Alzheimer's disease. (n.d.). Retrieved September 06, 2016, from http://www.accerapharma.com// Alzheimer's disease. (2015). Retrieved September 06, 2016, from http://www.mayoclinic.org/diseases-conditions/alzheimers-disease/diagnosis-treatment/departments-specialties/orc-20167134 Center for Alzheimer Research and Treatment. (2015, September 18). Retrieved September 06, 2016, from http://www.brighamandwomens.org/Research/labs/CART/Our_Mission.aspx Latest Alzheimer's Facts and Figures. (2016). Retrieved September 06, 2016, from http://www.alz.org/facts/ IP PositionPlease take a look at the following table to find a summary of current patents related to the design of our device: Fundability Worksheet ScoresCompetitors Customer Validation IP Position
| ||||||