BME100 s2014:T Group15 L3
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||
OUR TEAM
LAB 3A WRITE-UPDescriptive StatisticsThe data is analyzed in the following spreadsheet:
ResultsFor the temperature tests, five trials of inside temperatures were recorded in order to establish a baseline temperature reading for the experiment. After the resting temperature measurements were recorded, each group moved outside in order to record twelve more trials of temperatures and determine if there was a significant difference in the readings between the inside and outside trials. After the twelve were recorded, each group moved back inside in order to record the final set of five temperature readings. After each group recorded a total of twenty two temperature measurements, the data was shared between all groups in order to average the data sets for the resting temperature, outside temperature, and final temperature. It was found that the difference between the average of the inside and outside temperatures was not significant, with T-tests for both sets of data returning p-values of < .05. The inside mean temperatures recorded returned a p-value of .0004118, and the outside temperatures recorded returned a p-value of 1.349e-10. For the blood pressure tests, five trials of resting blood pressure and pulse measurements were recorded in order to establish a baseline blood pressure range to compare the active blood pressure results to. After the initial five, one subject walked briskly for a period of fifteen minutes in order to increase blood pressure. The subject then returned to the testing area in order to record twelve additional trials of blood pressure and pulse measurements. After all the data was collected, trial data was shared between all test groups and average values of blood pressure and pulse for both pre and post physical activity were compared. It was found that the difference between pre and post activity blood pressure readings was a little more than 1 bpm, and the T-test conducted on the data suggests that the difference was not statistically significant, having a p-value of < .05 for both pre and post activity blood pressure (.0226 and 2.527E-05 respectively). The pulse data suggested a slightly more significant discrepancy between pre and post activity readings, with a difference of about 3 bpm between the two sets. However, the differences for each set of pulse data was statistically significant, with T-tests for both sets returning values of >.05 (.354 and .191 respectively).
AnalysisAlthough our temperature sensor seemed to be faulty, it was determined by a p value that the difference between the thermometer and sensor was statistically insignificant. Therefore, the two were in proper working order. Because the T-tests determined that the data sets from the blood pressure experiments-- which posed both pre and post measurements between the cuff and wrist devices--did not have a difference that was statistically significant, we can conclude that the devices worked properly. Only in pre and post pulse measurements were displayed a real difference statistically. The p value being greater than .05 meant that there was a discrepancy between the readings of the watch device and pulseoximeter. While we are able to note there is a difference, we cannot be sure which device is accurate, if one of them is even accurate at all. Since the thermometer and the blood pressure cuff are the standard device for their measurements, and there was not a significant data difference between them and their opposing devices, it can be said that the sensor and the wrist device were accurate (data wise).
Summary/DiscussionFor the body temperature measurements the gold standard was definitely easier to use and more efficient in our group compared to the other sensor. Our sensor lost connectivity almost every five minutes. This made taking measurements more difficult and time consuming compared to the gold standard which took about thirty seconds and never had any technical difficulties. When comparing the results with the other groups, the sensor and gold standard were both very close on their temperature readings. A recommendation for the sensor would be to have a better connectivity or an easier way to be able to read the results. For the blood pressure measurements the gold standard and sensor were pretty equal. These tests were not as accurate as the body temperature ones though. The only other downfall of using the sensor over the gold standard was having two different devices, one on the wrist and one on the finger. A recommendation would be to get the sensor to where there is only one device being used instead of two.
LAB 3B WRITE-UPTarget Population and NeedElderly: 60-80 years old
Every day health - Heart Rate and Exercise Easy-use diabetes device Convenient help access for accidents - "Life Alert"
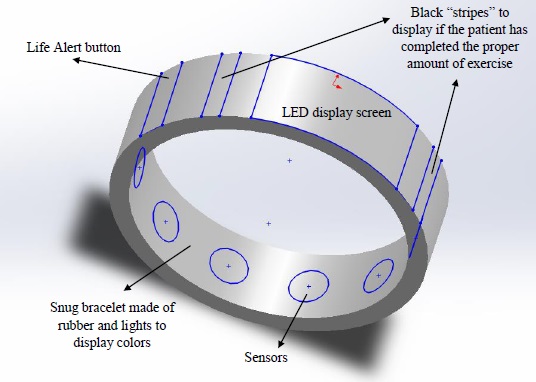
Device DesignThis device has several different components:
This device takes the shape of a bracelet so that it is convenient for users, they may use both their hands, and they will know exactly where their device is at all times. This device will also be waterproof so that it may be worn at al times, including while taking a shower, doing laundry, and hand washing. The bracelet will also have an initial neutral color of white/partially transparent but will change colors when the patient's glucose level has been checked, when the heart rate has reached a certain level for a given time, and when the heart rate is too high or low. There will also be vibrating incorporated and a "LifeAlert" button. This device is color and feel oriented so it is easy to use and understand. The parameters of the bracelet are input by the Dr. so each bracelet is individualized based on the patient's needs. The data collected is then sent to both an user friendly webpage for the patient to view as desired and to the Dr. or hospital so they may access the data and use it to better evaluate the patient.
The biggest component of this device is the non-invasive glucose level monitor. The patient no longer needs to poke themselves to get a blood sample for the important measurement of glucose levels. Not only is the device non-invasive it also has an easy LED screen for reading the levels as well as a color and vibrating component for an easy interpretation of the measurement. When the device is white everything is good. When it changes colors to a purple/blue it means the glucose levels are down, check the measurement to know how to proceed. If the device vibrates and turns a yellow/red then the glucose level is drastically low and immediate action is needed. When the device reflects a color of green it means the glucose levels are where they need to be. These measurements occur as the patient needs them, input by the Dr.
Heart Rate: The bracelet also continuously monitors the heart rate for exercise purposes as well as to help aid the Dr. when examining the patient. The black lines are displayed next to the screen when the heart rate has reached a pre-set value for a specific amount of time. These lines will disappear when the heart rate is lower than a certain heart rate for a given amount of time. This device will also vibrate and flash red when the heart rate is too low. If the heart rate is too low for a certain amount of time it will send out an alert for help and a built in GPS chip will help the medics locate the patient in need.
"LifeAlert": This device also has the added benefit of Life Alert.
Inferential StatisticsThe inferential statistics are proved in the following spread sheet.
GraphThe graphs are displayed in the following spread sheet:
|
|||||||