Droplet Microfluidics: T-Junction - Lina Wu
Introduction
Microfluidics systems most often take advantage of reduced length scales and volumes in continuous flow systems to perform functions and reactions more quickly or efficiently. The small dimensions nearly guarantee laminar flow, allowing for precise control of fluid locations and movement. However, both mixing and scaling up can be difficult.[1][2] Droplet microfluidics, in which the flow of a continuous liquid phase is broken up by droplets of another immiscible phase, is emerging as a superior method for performing well controlled and discrete functions. Unlike in continuous flow systems, each droplet can be independently controlled and manipulated, which allows for running many reactions in parallel without the need to scale up the device. Major applications for droplet microfluidics include using the well-controlled droplets as templates for the synthesis of materials such as microparticles or using droplets as microreactors in which chemical/biochemical reactions can be performed in parallel using very small reagent volumes.[3]
Droplet Generation

Droplet formation is controlled by the formation and deformation of the liquid-liquid interface between the two immiscible phases. There are a variety of different geometries and methods that can be utilized in microfluidic systems to create droplets with different sizes and frequencies, as shown in Figure 1. The simplest geometry is the T-junction (Figure 1a.i), which is also the classic example of passive droplet generation utilizing hydrodynamic or gravitational forces. Other passive generation methods consist of co-flow, flow focusing, or step geometries that can exhibit different droplet breakup modes such as tip-streaming and tip-multi-breaking. There are also active methods of droplet generation that rely on external forces such as mechanical agitation, electric fields, magnetic fields, or lasers. In the case of 2D microfluidic devices with no external forces, droplet generation is governed by inertial forces, viscous forces, and capillary forces. When the two liquid phases meet at the junction, they form an unstable liquid-liquid interface that is deformed and eventually broken up to form discrete droplets.[4]
T-Junction

The T-junction is one of the most commonly used geometries for microfluidic droplet formation (Figure 2). This geometry is easy to fabricate, operate, and control and creates consistent droplets of relatively high monodispersity at a high rate.[1] In this configuration, the dispersed phase is pumped into the continuous phase orthogonally. As the dispersed phase enters the continuous, shear forces elongate the head of the dispersed phase until a segment eventually separates and relaxes into a sphere or plug shape due to interfacial tension.[2] While a single T-junction is the most simple geometry for the microfluidic formation of droplets, they can be combined to create more complex droplets, or manipulate droplets to precisely perform a specific sequence of steps, as can be seen in Figures 2a.v and 2a.vi.
Droplet Formation

There are several dimensionless parameters that dictate droplet generation in microfluidic T-junctions. The most important of these is the capillary number (Ca), which represents the ratio of viscosity to interfacial tension. Viscous forces dominate at high Ca while interfacial tension effects dominate at low Ca. Droplet diameter decreases as Ca increases, with spherical droplets forming at low Ca and long liquid plugs at high Ca (Figure 3). Regardless of the size of the liquid plug, there remains a thin layer of the continuous phase between the discontinuous phase and the walls at all times. If the channels walls are wet by the discontinuous phase in the junction/droplet generation area, droplet formation will be unstable. Using a non-wetting material like Teflon or coating the walls of other materials (i.e. PDMS) in the junction region can prevent wetting of the walls and improve the stability of droplet generation. Additionally, the ratio of the flow rates and viscosities of the continuous and dispersed phases and the wettability of the continuous phase will impact droplet formation. There are three breakup modes observed in droplet formation with T-junctions that depend on relative flow conditions for the dispersed and continuous phases: squeezing, dripping, and jetting.[5]

Within a certain droplet breakup mode, the size of the microfluidic droplets is dependent on material properties as well as relative flow rate. Therefore, the size of the droplets is generally changed by altering the relative flow rates of the continuous and discontinuous phases. If the flow rates of the two phases are gradually changed such that their relative flow rates differ but the total flow rate stays constant, the droplets will also gradually change their size. This allows for the testing of a variety of different droplet sizes and conditions while keeping overall conditions constant. If there are multiple streams feeding into the discontinuous phase, changing their relative flow rates allows for gradually altering the internal composition of the droplet while keeping flow rate and droplet size constant. Different reagents can first be aliquoted into droplets that act as the reagent feed stream to further increase throughput of screening (Figure 4).[6] Also, since the changing conditions are automatically generated by the changing flow rates and each droplet acts as a separate experiment, the throughput for such experiments is much higher than can be done with macro-scale experiments or single phase microfluidics.
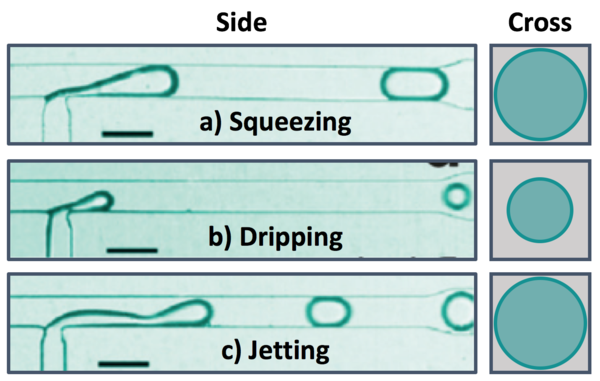
Squeezing
At low capillary numbers, droplets are formed in squeezing mode, in which a pressure gradient formed across the droplet as it is formed (Figure 5a).[1] The dispersed phase protrudes into the main channel and the pressure decreases between the interface and the opposite wall as the continuous phase must speed up around the protrusion until the dispersed phase almost completely obstructs the flow of the continuous phase. This causes pressure to build up behind the junction in the continuous phase that eventually get high enough to break off droplet. Droplets generated in the squeezing mode travel through the channel as a plug confined by the channel walls.[4]
Dripping
As the relative flow rate of the continuous phase is increased and the capillary number increases, droplet generation transitions from squeezing mode to dripping mode (Figure 5b). In dripping mode, viscous shear stress and interfacial tension compete.[1] Because of the relatively reduced flow rate of the dispersed phase, the droplet fluid is broken up by the continuous fluid before the droplet can reach the opposite wall and form a liquid plug. Droplets formed in dripping mode are spherical and have diameters smaller than that of the channel.[4]
Jetting
When the flow rates of both the continuous and dispersed phases are increased, droplets are formed in jetting mode, where the dispersed phase enters the main channel as a liquid jet that is eventually broken up into droplets due to instability of the interface (Figure 5c).[4] Droplets formed in the mode can either travel through the channel as a spherical droplet or a liquid plug, depending on the relative flow rates of the continuous and dispersed phases.
Fabrication
T-junctions can be integrated into a microfluidic device to produce droplets that are immediately used, or can be used as a connecter for fluid tubing. For on-chip T-junctions, there are separate inlets for the continuous and discontinuous phases and the fluids will meet, interface, and form droplets at the junction of the two chambers. Most often, these devices are made with PDMS due to its transparency and high resolution in replica molding. Generally, on-chip generation is preferred for more precise control over droplet properties, the formation of more complex droplets, or when droplet generation is integrated into a larger process contained on the chip. Similarly, when separate tubes of the continuous and discontinuous phase are connected with a T-shaped tubing connector, the fluids will interface and form droplets that will be pumped through the outlet tube. These droplets can then be transported to wherever they next need to be, which makes this off-chip T-junction ideal for modular systems. This method of droplet generation is less controllable but also more robust, can be used for longer periods of time, and can withstand higher pressures (depending on the material of the tubing) than traditional PDMS devices.[7]
Applications
Chemical Reactions

Using droplets as microreactors allows for the performance of many different well-controlled reactions using very small amounts of material. The small length scales allow for much more efficient heat and mass transfer, which are typically problematic for macro-scale batch reactions. Microfluidic droplet reactions are also much easier to control through varying droplet size, flow rate, and temperature.[4] The small volumes and confinement of the reaction also limit exposure to dangerous reagents or products. Due to the high throughput of droplet generation and the ability to independently control each droplet, droplet microfluidics can also be used to more rapidly and efficiently generate and screen compound libraries for drug discovery of both small molecule and large molecule drugs.[2]
Song et al. utilized an on-chip T-junction to follow the titration reaction of the clotting agent argotroban and blood (Figure 6). The aqueous phase reagents are pumped into droplets in the main channel where they are then mixed convectively with a winding sinusoidal channel. After incubation, the droplets are fused with droplets of calcium chloride, initiating the clotting of the blood. After another mixing segment, the remainder of the long channel is used to observe the progression of clotting using microscopy.[8] One advantage of using T-junction droplet generation (and other methods that generate one droplet at a time) is that the linear distance along the channel is a linear function of time, assuming a constant flow rate. Therefore, the reaction can easily and accurately be monitored as it progresses with time.
Materials Synthesis

The well-controlled and monodisperse droplets created with microfluidic T-junctions are commonly used as templates for the fabrication of materials with specific geometries. A reactive mixture can be dispensed as the dispersed phase into an inert carrier that will react over time or with the application of some external stimuli, such as heat or light. Figure 7 shows an example of this type of microfluidic system that generates nano-sized metal-organic frameworks (MOFs) in droplets produced with a T-junction.[9] This system is fundamentally the same as that of the argatroban titration where the aqueous reagents are mixed within droplets and react while traveling through the device either just time or heat. The reaction can also be accurately followed over time because the length will correspond to the residence time. Alternatively, different components can be dissolved in different phases and react as the component diffuse into/out of the droplet. Different droplets with different components can also be generated separately and mixed together to initiate the reaction, like with the calcium chloride in the argatroban system.[1]

Another advantage to using microfluidics for nanoparticle generation is that the particle morphology can be much more easily manipulated than by conventional methods. There are many ways in which to alter the final nanoparticle shape, but the simplest is to deform the droplet into a specific shape while the reaction occurs by altering the channel geometry, as seen in Figure 8.[10] Changing the droplet microenvironment can also change both its shape and its internal structure, allowing for even better control on particle morphology.
Encapsulation


Droplet microfluidics can be used to isolate and encapsulate individual cells for manipulation or analysis. Since the concentration of cells in solution is not completely uniform while droplets are formed at a fixed rate, the number of cells in each droplet follows a Poisson distribution. However, Edd et al. passed the cell suspension through a microchannel with a high aspect ratio, causing them to align to either side of the channel. Droplets formed from this ordered stream then will have a nearly constant number of cells encapsulated if the rate of droplet formation is matched to the rate of cell flow (Figure 10).[11] While Edd et al. used a flow-focusing geometry in this instance, a T-junction could be utilized for the same task if the cells were aligned to one side of the channel instead. The process of encapsulation of a cell using a T-junction is shown in Figure 9.[12] The microenvironment of the droplets with encapsulated cells can then easily be controlled and changed, so individual cells can be processed in a highly controllable manner that is difficult to achieve through other methods.[13] Alternatively, droplets can be utilized as artificial cells in the synthesis of biomolecules such as proteins or DNA. Like individual cells, individual DNA templates can be encapsulated and processed to create a higher throughput method of directed evolution for either proteins or DNA/RNA.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 R. Seemann, M. Brinkmann, T. Pfohl, S. Herminghaus, Rep Prog Phys, 2012, 75. http://dx.doi.org/10.1088/0034-4885/75/1/016601
- ↑ 2.0 2.1 2.2 S. Teh, R. Lin, L. Hung, A. P. Lee, Lab Chip, 2008, 8, 198-220. http://dx.doi.org/10.1039/b715524g
- ↑ P. S. Dittrich, M. Jahnz, P. Schwille, ChemBioChem, 2005, 6, 811–814. http://dx.doi.org/10.1002/cbic.200400321
- ↑ 4.0 4.1 4.2 4.3 4.4 P. Zhu, L. Wang, Lab Chip, 2017, 17, 34-75. http://dx.doi.org/10.1039/c6lc01018k
- ↑ H. Liu, Y. Zhang, J App Phys, 2009, 106, 034906. https://doi.org/10.1063/1.3187831
- ↑ L. Li, D. Mustafi, Q. Fu, V. Tereshko, D. L. Chen, J. D. Tice, R. F. Ismagilov, Proc Natl Acad Sci USA, 2006, 103, 19243−19248. https://doi.org/10.1073/pnas.0607502103
- ↑ L. Shang, Y. Cheng, Y. Zhao, Chem Rev, 2017, 117, 7964-8040. https://doi.org/10.1021/acs.chemrev.6b00848
- ↑ H. Song, H. Li, M. S. Munson, T. G. Van Ha, R. F. Ismagilov, Anal Chem, 2006, 78, 4839-4849. https://doi.org/10.1021/ac0601718
- ↑ M. Faustini, J. Kim, G. Jeong, J. Y. Kim, H. R. Moon, W. Ahn, D. Kim, J Am Chem Soc, 2014, 135, 14619-14626. https://doi.org/10.1021/ja4039642
- ↑ . S. Xu, Z. Nie, M. Seo, P. Lewis, E. Kumacheva, H. A. Stone, P. Garstecki, D. B. Weibel, I. Gitlin, G. M. Whitesides, Ange Chemie, 2005, 44, 724-728. https://doi.org/10.1002/anie.200462226
- ↑ J. F. Edd, D. Di Carlo, K. J. Humphry, S. Koster, D. Irimia, D. A. Weitz, M. Toner, Lab Chip, 2008, 8, 1262-1264. https://doi.org/10.1039/b805456h
- ↑ A. E. Sgro, P. B. Allen, D. T. Chiu, Anal Chem, 2007, 79, 5845-4851. https://doi.org/10.1021/ac062458a
- ↑ A. Huebner, M. Srisa-Art, D. Holt, C. Abell, F. Hollfelder,A. J. Demello, J. B. Edel, Chem Commun, 2007, 1218-20. http://dx.doi.org/10.1039/b618570c
