IGEM:Harvard/2006/Adaptamers/Notebook/2006-8-17
8/17
Detection of binding of thrombin to streptavidin using antibodies
Secondary antibody information:
http://www.rockland-inc.com/commerce/catalog/product.jsp?product_id=11755&czuid=1155835467300
I think I'll use some shorthand.
- Abbreviations
SMB: streptavidin magnetic beads
MB: carboxylated magnetic beads
HT: human thrombin
BB: Bittker buffer
W: Wash
E: Elution
- Conditions:
A35 (Adaptamer 35)
A15 (Adaptamer 15)
DS (Double stuff: 320 pmol thrombin vs. 160 pmol thrombin)
NT (No thrombin)
1S (Primary and secondary antibodies added in one step; incubation for an hour instead of two 30 minute sessions)
JB (just magnetic beads; no streptavidin has been coupled to them)
- Specific Procedure
A35, A15, DS, NT, 1S: 200 uL .4% SMB -->wash (BB) --> 10 uL
JB: 15 uL 10% MB -->wash (BB) --> 10 uL
A35, DS, NT, 1S, JB: +50 pmol A35-->15uL
A15: +50 pmol A15-->Bittker Buffer-->15 uL
A35, A15 NT, 1S, JB: +160 pmol thrombin-->16uL
DS: +50 pmol A15-->Bittker Buffer-->16 uL
All: 3X wash with Bittker Buffer-->10 uL
A35, A15, DS, NT, JB: 30 minute incubation with 5 uL 200 ug/mL anti-thrombin primary antibody, 2X wash with 1 mL Bittker Buffer-->10 uL, 45 minute incubation with 1 uL 1 mg/mL rabbit anti-goat secondary antibody.
1S: 105 minute incubation with 5 uL 200 ug/mL anti-thrombin, 1 uL 1 mg/mL thrombin antibody.
All: 2X wash in 1 mL Bittker buffer--> 10 uL; stored overnight
Western blots
The above assay may suffer from the numerous wash steps and the long (by comparison with the time of washes) incubation with primary and secondary antibodies. Therefore, another option is to follow the protocol last week, running a Western Blot to detect proteins, which should be more sensitive than the staining we've used in the past. Additionally, we will concentrate our samples more, which might have led to the higher signals seen using magnetic beads.
- General setup
Incubate streptavidin beads, adaptamer, and thrombin, then wash and elute with free streptavidin
- More abbreviations
SAB: streptavidin agarose beads TAB: thrombin agarose beads
- Conditions
A20: adaptamer = A20
A50: adaptamer = A50
2X: 320 pmol thrombin vs. 160 pmol thrombin, 160 pmol A20 instead of 80 pmol A20 added.
ND: No DNA added.
TB: Use thrombin beads instead of streptavidin beads
- Specific procedure
200 uL SAB (didn't have enough SMB) and 50 uL TAB washed in Bittker buffer 3X and reconstituted.
A20, A50, DS, S20: 50 uL SAB spun down (14000 rpm); supernatant removed to greatest extent possible; 10 uL Bittker buffer added.
TB: 50 uL TAB spun down; supernatant removed; 10 uL Bittker buffer added
A20, TB: 8 uL 10 uM A20 added.
2X: 16 uL 10 uM A20 added.
A50: 8 uL 10 uM A50 added
ND: 4 uL Bittker buffer added.
A20, A50, TB, S20: 1 uL 160 uM thrombin added.
2X: 2 uL 160 uM thrombin added.
All: Pipetted up and down, shaken on Labquake for 30 minutes. Samples spun down and supernatant removed (wash); washed 2x in 1 mL Bittker buffer. 25 uL 1 mg/mL streptavidin added, put back on shaker for 45 minutes. Samples spun down and supernatant removed (elution).
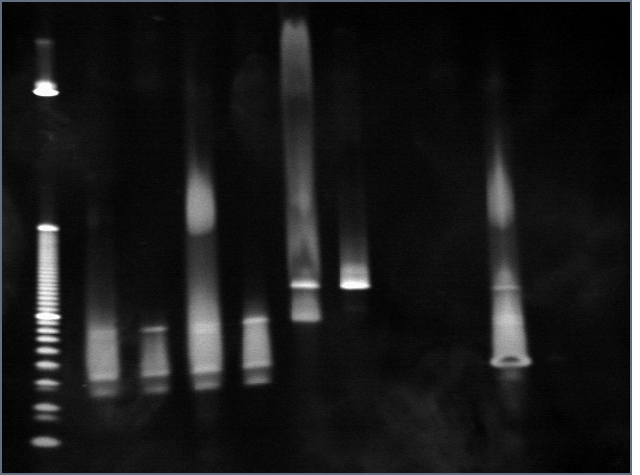
DNA gel:
8 uL of wash and elution were run on a 4-20% PA gel at 200V for 30 minutes in TBE.
- DNA
| Lane | Condition |
| 1 | 10bp ladder |
| 2 | A20 W |
| 3 | A20 E |
| 4 | 2X W |
| 5 | 2X E |
| 6 | A50 W |
| 7 | A50 E |
| 8 | ND W |
| 9 | ND E |
| 10 | TB W |
| 11 | TB E |
- Western Blot
8 uL wash and 16 uL elution was run on a 12% PA gel for 90 minutes at 125 V. Western blot was carried out as according to here up to blotting stage; blotting blotting machine was run at 2V for 8 hours overnight.