Importance of 3D Cell Culture - Maximilian Marek
Introduction: 2D vs. 3D Cell Culture
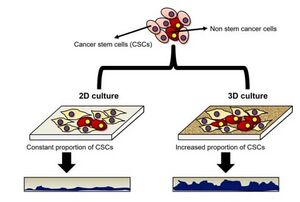
The growth patterns of different cells between 2D and 3D cell culture are widely different (Figure 1). Two-dimensional cell culture has been a traditional method used since 1907 by Ross Harrison when trying to observe nerve fibers. Known for its short amount of time, cheapness, and ease of reproducibility, this technique has been carried out since and has greatly advancing the scientific field. With some cell lines, it is better to use 2D cell culture, specifically when trying to perform simple cell culture techniques such as cell splitting. However for cancer cells, the usage of 3D cell culture is considered significantly better. One of the advantages is an actual mimicry of an in vivo environment with different cell-cell and cell-environment factors impacting growth and formation. As such, you end up with asymmetrical tumors which have similar morphology to those of an in vivo experiment. (1) In addition, 3D cell culture can keep the phenotype characteristics of cells which is important when dealing with cells that function different depending on their phenotype, such as macrophages. Without this, experiments based on different phenotypes or morphologies could not occur. The main disadvantages of 3D cell culture are the increased costs, waiting time, and difficulty in reproducibility. The process of forming a 3D cell culture model of a tumor may take several days compared to the rapid cancer cell growth in 2D cell culture, and since these are 3D reproducing the exact shape is nearly impossible. However, for performing certain experiments, 3D cell culture is a must and is best represented in experimental microfluidic devices (2).
Introduction: Micro-scale application of 3D Cell Culture
The usage of three-dimensional (3D) cell culture in microfluidic devices was first published in 2009 in a collaborative study from MIT. It had already been established that 3D cell cultures were considerably better than 2D cell cultures due to their inclusion of the extracellular matrix (ECM) and other growth factors. Upon this initial study, it was found that the formation of these 3D spheroids showed more accurate growth pattern and treatment results compared to their 2D counterpart. However, a different set of considerations must occur for 3D cell culture at the microfluidic scale, such as the materials used and unintentional oxidation (3).
These devices are typically made with PDMS consisting of several different micropillars. These micropillars are evenly spaced such that there is an increase of cell-to-cell interactions and a decrease in physical shear stress. (2) PDMS is a material that has always been considered the norm for microfluidic devices and 2D cell culture. As such, to prove the 3D model, PDMS devices were also fabricated with these microarrays. These devices consist of several inlet and outlet streams for the injection of cells and removal of byproducts, respectively. In addition, an agarose gel or some other scaffold must be coated on the device where the cells would be present in order for proper growth/behavior to occur. Otherwise a 3D cell model could become deformed that can skew experimental results. As such, the cells must have a longer doubling time in order for proper experiments can occur for adding any form of therapeutic. Another method for loading cells onto the microfluidic device involves directed scaffolds with growth factors, such as collagen IV attached. This would allow for continuous cell growth after several passages (4).
Device Materials
There are several different materials that are used for microfluidic devices that impact overall growth and behavior of 3D cell culture (5).
PDMS
PDMS is one of the most commonly used materials in microfluidics due to its wide range of properties. PDMS is an elastomer that has a high surface area to its bound substrate which is important in its soft lithography process in order to hold the desired device template. In specific to 3D cell culture, PDMS is chemically inert, isotropic, and can allow for observation of light down to 300 nm. Since the device is chemically inert, there is no negative reactions that could occur when trying to apply different types of treatments on the actual device or that would alter the device to impact the cells. Since the device is isotropic, there would be no difference in cellular conditions throughout the device such that one side doesn't favor growth over the other. With visibility of substances at 300 nm or above, this is critically important in cell culture for observing the fluorescence of novel treatments. Since green and red fluorescence are above 300 nm, proper visualization can occur in order to determine if the treatment properly works (5).
Polystyrene
There are some instances where PDMS cannot be used however due to potential impacts on cell proliferation due to the absorption of growth factors from the scaffold and interference with the cell culture media. In addition, in the case of therapeutics testing, this can skew the results such that the therapeutic can be absorbed into the surrounding material rather than target the cells. From a design standpoint, a polystyrene based microfluidic does not require micropillars as PDMS does since polystyrene is hydrophobic to the point where interactions between wells would not occur. Micropillars are often used in PDMS devices in order to make sure that there is no mixing between different wells or culture media with cells if needed. Polystyrene however is a commonly used material in standard 2D cell culture and as such can be seen as a recommended substitute for a microfluidic device if there are experimental cytotoxicity materials. However at this time, it is unknown how long a 3D cell culture environment can survive in a polystyrene device as the current study only shows a 4-day long experiment. But it does provide the possibility that polystyrene would be able to determine different gas testing abilities, such as with cigarette smoke (6).
Glass/Silica
Glass is often used now as a base for polymeric microfluidic devices, such as PDMS. However, glass devices in the past have shown with an increase of visibility and imaging quality of the 3D cell culture systems based on the fluorescence tagging discussed earlier. There have also been studies using silica for 3D cell cultures, mostly for nutrient delivery to different cells. However, due to the fragility of the glass devices and the cost of the silica devices, these processes are rare with the cheapness of polymeric devices (5,7).
Paper-Based
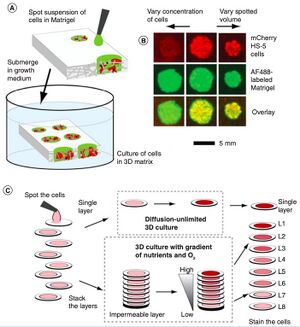
Considered a much cheaper option for microfluidic devices to sustain 3D cell culture, paper microfluidics was first established by the Whitesides group. This method includes chromatography paper that is stacked and contains suspended cells (Figure 2). Cells are grown in a growth media before being spotted onto the paper, which is then stacked. This best modifies the oxygen gradient that occurs with 3D cell cultures where there is a higher concentration at the surface level than there is at the core of the 3D structure (8).
Air Bubble Issues
One of the current challenges with 3D spheroid formation is air bubble formation. This can develop due to poor sealing and constant circulation of fluids. One of the problems with air bubbles is the increased amount of oxygen within the cell culture which will lead to reduction in stability and flow resistance in the normally Nitrogen environment. Different culture flows were tested at media concentrations ranging from 10% to 40% with these endpoints providing the lowest amount of bubble formation. This is a major consideration with 3D cell culture since proper growth needs to occur for accurate results. (9)
References
1) Gupta, N. et al. Bioengineering & Translational Medicine 2016, 1 (1), 63-81. https://doi.org/10.1002/btm2.10013
2) Kapalczynska, M. et al. Archives of Medical Science 2018, 14 (4), 910-919. https://doi.org/10.5114/aoms.2016.63743
3) Sudo, R. et al. Biomicrofluidics 2014, 23 (1), 2164. https://doi.org/10.1096/fj.08-122820
4) Kim, M. S.; Hwang, H., Choi, Y. S.; Park, J. K. The Open Biotechnology Journal 2008, 2, 224-228. https://doi.org/10.2174/1874070700802010224
5) Xia, Y. & Whitesides, G. M. Angewandte Chemistry International Edition 1998, 37, 550-575. https://doi.org/10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G
6) Chan, C. Y.; Goral, V. N.; DeRosa, M. E.; Huang, T. J.; & Yuen, P. K. Biomicrofluidics 2014, 8 (1), 046505. https://doi.org/10.1063/1.4894409
7) Li, X.; Valadez, A. V.; Zuo, P.; Nie, Z. Bioanalysis 2012, 4 (12), 1509-1525. https://doi.org/10.4155/BIO.12.133
8) Derda, R. et al. Proceedings of the National Academy of Sciences of the United States of America 2009, 106 (44), 18457-18462. https://doi.org/10.1073/pnas.0910666106
9) Park, D. H.; Jeon, H. J.; Kim, M. J.; Nguyen, X. D.; Morten, K.; & Go, J. S. Journal of Micromechanics and Microengineering 2018, 28 (1), 045001. https://doi.org/10.1088/1361-6439/aaa877
