Lung on a Chip
Lung on a Chip
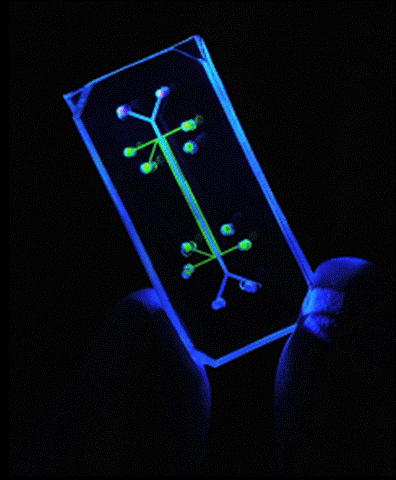
The Lung on a Chip is a microfluidic device which serves as a robust model of a human physiological lung system. It was developed by Dongeun Huh, Donald Ingber and other members of the Wyss Institute at Harvard University in Cambridge, Massachusetts. The successful construction and initial experimentation with the model were released in Science in June 2010.
Motivation
Two key motivating factors behind the development of the Lung on a Chip are the high costs associated with animal testing and clinical trials which are currently required to bring novel drugs and compounds to market, as well as the desire to develop systems that more accurately model human physiological systems without endangering human test subjects or patients.
Costs of Drug Development
A study released in 2003 in the Journal of Health Economics estimated the total cost in real dollars spent by pharmaceutical companies to bring single new drug from discovery, through development, animal testing and clinical trials at $802,000,000 [1]. The much disputed study was conducted by members of the Tufts Center for Drug Development at Tufts University in Medford, Massachusetts. The members of the study estimated this cost based on dollar value in the year 2000, and that number may even have risen to closer to $1.3 or $1.7 Billion US Dollars [2]. These are staggering costs which force pharmaceutical companies to charge prohibitively high costs for many of their treatments which some patients, particularly those without adequate health insurance, simply cannot afford. A pharmaceutical advertisement during the mid-2000’s illustrated this strongly with the quote: “Today’s medicines finance tomorrow’s miracles.” [2]
Animal Testing and Tissue Culture Models
Currently animal testing is a widespread standard of observing in vivo behavior of new drugs, therapies and compounds across the medical, pharmaceutical and research communities. Animal models can provide valuable data, but come at great financial cost and loss of life to animal test subjects. Additionally, animal testing as a model of human reaction to compounds is often not a strong indicator of similar results in human subjects due to stark differences in physiology and animal behavior. Two-dimensional culturing methods have been increasingly successful at growing and maintaining human cell types. These have been utilized as methods of screening drugs and other novel compounds especially for toxicity and related studies. However, two-dimensional models are poor approximations of complex three dimensional whole organ physiological systems due to the limited ability for cells to interact and organize in two dimensions.
Three dimensional tissue models such as hydrogel or other cell seeded scaffolds systems can provide greater insight into cellular behaviors due to increased cell-cell signaling and migration that can take place in three dimensional cultures. These systems have been demonstrated by various groups to model various cell and tissue types; however their long term viability is limited by transport of nutrients, oxygen and other vital compounds to cellular survival in a full organ or tissue system due to the inability to successfully incorporate vessels approaching natural occurring vasculature[3].
Naturally Occurring Lung Tissue
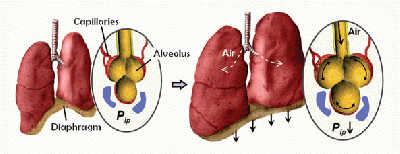
Natural lung tissue is comprised of a highly bioactive interface between air breathed in through the lungs and the circulatory system. Alveoli make up a large portion of the lungs and expand and contract elastically during normal respiration. Blood vessels snake their way across the surface of the alveoli to maximize the surface area of the gas-liquid interface. It is across this interface that oxygen is up taken by the circulatory system’s red blood cells and carbon dioxide waste is transferred back into the alveoli to be breathed out of the body by the lungs. This is a dynamic, complicated system comprised of diffusive, mechanical, and structural forces that the lung epithelium cells and circulatory endothelium cells involved rely on to carry out their required gaseous exchange reaction.
[From the next section on, the information was summarized from reference [4], the initial Lung-on-a-Chip Science article, published in June of 2010]
Construction of the Lung on a Chip
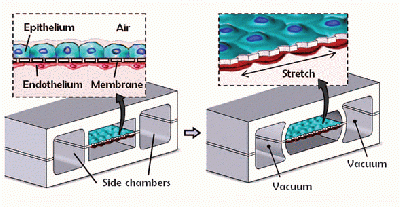
The lung on a chip is constructed primarily of poly(dimethylsiloxane) (PDMS), a soft, partially elastic polymer, that has been patterned through soft lithography techniques typical of nanotechnology and microfluidic devices. Two pieces of a PDMS slab are patterned with three different channels about ten microns in width. These two PDMS slabs are sandwiched around a thin PDMS membrane that has been patterned with pentagonal pores. The three pieces of PDMS are fused together to create three microfluidic channels that are divided down the middle by the porous PDMS membrane. The outermost channels are exposed to a PDMS etching compound that etches away part of the exposed PDMS surface, including the entire membrane. These outermost channels are the vacuum channels that will cause the PDMS chip to expand and contract similar to natural lung tissue. The middle channel that remains with its porous membrane intact is where the gas exchange takes place. Human lung epithelial cells are seeded onto the top layer of the chip and they adhere to the sides of this channel including the porous PDMS membrane while they are exposed to air. On the opposite side of the membrane, human endothelial cells are seeded on to the membrane and channel surface while a blood modeling media is flowed through. The vacuum channels will be connected to a computer driven vacuum pump which will cause the central channel and it’s membrane to expand and contract, while microfluidic pumps model the flow of air and blood through the central channel on either side of the membrane.
Initial Experiments to Asses Model Efficacy
Two experiments were carried out using the lung on a chip model and its response compared to results in animal models.
Bacterial Lung Infection
E.Coli bacteria were introduced into the air (lung) side of the membrane to model a bacterial lung infection in a living organism. White blood cells were added to the blood modeling media on the other side of the membrane to determine the efficacy of an immune response. The white blood cells on the blood side of the membrane were able sense the bacterial infection, attach themselves to the endothelial cells and move through the pores in the PDMS membrane to attach and attack the bacterial cells. This is exactly how immune responses occur with bacterial infections in animal models.
Inhalation of Nanopatricles
The lung on a chip was also used to observe the modeled inhalation of nanoparticles. A nanoparticle solution was introduced to the lung side of the device and then allowed to dry to model the deposition of nanoparticles on the surface of the alveoli that would come as a result of their inhalation by an organism. The lung on a chip model showed that the contraction and expansion of the porous membrane at the air liquid interface facilitated the transport of these nanoparticles across the interface and into the blood side of the device, once again effectively reproducing the uptake phenomenon exhibited in mouse models.
Other Organ on a Chip Models and Their Advantages
The initial development of the Lung on a Chip model is proof of concept that similar microfluidic devices could be created to model other organ systems. Many of the organ systems within the body involve interfaces between the circulatory system and other organ systems that can be similarly recreated based on the success of the cell lined PDMS porous membrane. If other “organ on a chip” systems are created, the hope is that ultimately several of the different devices could be interconnected to create models of multi-organ systems and analyze how these “organs” may interact within each other on the basis on infection, response to drug compounds or other tests based around these types of systems.
References
[1] DiMasi, J.A., Hansen, R.W., Grabowski, H.G., Journal of Health Economics. 2003. 22. 151-185
[2] Canadian Medical Association. Journal of the Canadian Medical Association. ”Drug Devlopment Cost Estimates Hard to Swallow”. 2009. 180 (3) 279-280.
[3] Pampaloni, F., Reynaud, E.G, Stelzer, E.H.K., Nature Reviews: Molecular and Cell Biology. 2007 8. 839-845
[4] Huh, D., Matthews, B.D., Mammoto, A., Montoya-Zavala, M., Hsin, H.Y., Ingber, D.E., Science. 2010. 328. 1662-1668
[5] Wyss Institute. “Lung on a Chip.” 2010. Accessed 19Apr12. http://wyss.harvard.edu/viewpage/240/
[6] Wyss Institute. “Organs on a Chip.” 2010. Accessed 19Apr12. http://wyss.harvard.edu/viewpage/293/
