Physics307L:People/Mondragon/Notebook/071121
calibration
SJK 21:45, 1 December 2007 (CST)

You missed the fact that you were supposed to rotate the prism to correct for this mis-calibration! Your data would be much better in that case. (These methods may be appropriate after you've rotated the prism to the best of your abilities. (Also, did you account for the backlash?)
using mercury to calibrate. center of slit, measuring clockwise
| color | actual wavelength | measured wavelength |
|---|---|---|
| red | 690.75nm | 634nm |
| yellow | 579nm | 545nm |
| yellow | 577nm | 542nm |
| green | 546.1nm | 525nm |
| violet | 435.8nm | 422nm |
so the calibration curve is [math]\displaystyle{ real\,wavelength=1.204205321\times measured\,value-76.8339591 }[/math]
the hydrogen spectrum
SJK 21:49, 1 December 2007 (CST)

Did you really only take one measurement for each color? (After calibrating correctly (above)), you need to take many "independent" measurements at each color to minimize uncertainty.
| color | Measured Value | Actual Wavelength (nm) | Actual Wavelength (m) | 1/wavelength | n | [math]\displaystyle{ 2^{-2}-n^{-2} }[/math] | Rydberg Constant |
|---|---|---|---|---|---|---|---|
| red | 604 | 650.5060546 | 6.50506E-07 | 1537264.708 | 3 | 0.138888889 | 1.1068306E+07 |
| blue green | 466.5 | 484.927823 | 4.84928E-07 | 2062162.558 | 4 | 0.1875 | 1.0998200E+07 |
| violet | 421 | 430.1364809 | 4.30136E-07 | 2324843.496 | 5 | 0.21 | 1.1070683E+07 |
| faint violet | 400.2 | 405.0890102 | 4.05089E-07 | 2468593.259 | 6 | 0.222222222 | 1.1108670E+07 |
mean value for Rydberg constant=[math]\displaystyle{ 1.1061*10^{7}\,\mathrm{m}^{-1} }[/math] standard deviation [math]\displaystyle{ 4.6*10^{4}\,\mathrm{m}^{-1} }[/math]
CALCULATIONS
heres the excel file File:L and T Balmer series.xlsx Section above has numbers from the excel file.
here is a more careful analysis
Linefit says the calibration data fits the line [math]\displaystyle{ actual\,wavelength=(1.204205 \pm 0.042636)*measured\,wavelength-(76.833959 \pm 22.932003)nm }[/math]
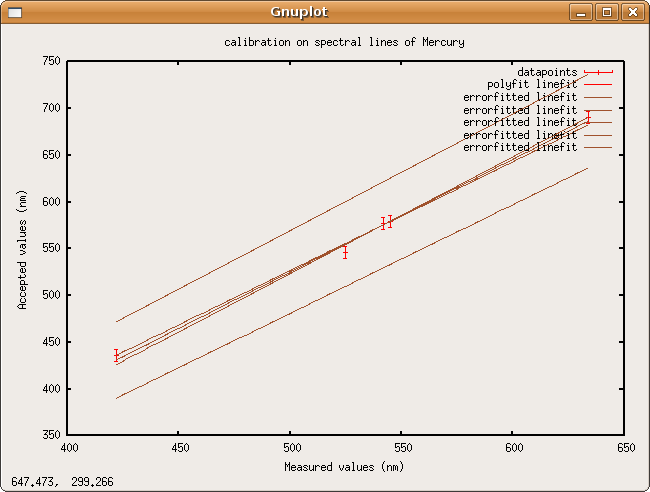
Error propagation
No data on measurement error was ever taken, so, for any measurement of wavelength the error on the calculated actual value will be
- [math]\displaystyle{ actual\,wavelength\,error=0.042636*measured\,wavelength+22.932003nm }[/math]
The calculated Rydberg constant is related to the measured wavelength value thusly
[math]\displaystyle{
\begin{align}
\frac{1}{\lambda} &= R_\mathrm{H}\left(\frac{1}{2^2} - \frac{1}{n^2}\right), n=3,4,5,...\\
R_\mathrm{H} &= \frac{1}{\left(\frac{1}{2^2} - \frac{1}{n^2}\right)\lambda}\\
R_\mathrm{H} &= \frac{1}{\left(\frac{1}{2^2} - \frac{1}{n^2}\right)(m*\lambda_m+b)}\\
\end{align} }[/math]
Where [math]\displaystyle{ \lambda_m }[/math] is the wavelength measured by the instrument, [math]\displaystyle{ m }[/math] is the slope of the calibration curve, and [math]\displaystyle{ b }[/math] is the y intercept of the curve. Because of the uncertainty in the fit, the calculated value of the Rydberg constant will have uncertainty.
[math]\displaystyle{ \Delta R_\mathrm{H} = \frac{1}{\left(\frac{1}{2^2} - \frac{1}{n^2}\right)(m*\lambda_m+b)^2}(\Delta m*\lambda_m+\Delta b)= R_\mathrm{H} \frac{\Delta m*\lambda_m+\Delta b}{m*\lambda_m+b}=R_\mathrm{H} \frac{\Delta \lambda}{\lambda} }[/math]
The uncertiainty in RH is just RH times the relative uncertainty in the calculated wavelength.
| color | n | Accepted Wavelength (nm) | Measured Value | Calulated Actual Wavelength (nm) | Calculated Error(nm) | Relative Error | Actual Wavelength (m) | Rydberg Constant | Rydberg uncertainty |
|---|---|---|---|---|---|---|---|---|---|
| red | 3 | 656 | 604 | 650.506 | 48.684 | 0.074840 | 6.50506E-07 | 1.10683E+07 | 8.284E+05 |
| blue green | 4 | 486 | 466.5 | 484.928 | 44.822 | 0.088305 | 4.84928E-07 | 1.09982E+07 | 9.712E+05 |
| violet | 5 | 434 | 421 | 430.136 | 40.882 | 0.095044 | 4.30136E-07 | 1.10707E+07 | 1.0522E+06 |
| faint violet | 6 | 410 | 400.2 | 405.089 | 39.994 | 0.098731 | 4.05089E-07 | 1.11087E+07 | 1.0968E+06 |
Average for Rydberg constant= [math]\displaystyle{ 1.10592*10^7\,\mathrm{m}^{-1} }[/math]. Uncertainty of average = [math]\displaystyle{ 9.64*10^5\,\mathrm{m}^{-1} }[/math]. Accepted value = [math]\displaystyle{ 10 967 758.341 \pm 0.001\,\mathrm{m}^{-1} }[/math]