User:Mar/Notebook/2007-6-19
migration from GE to cortical fractions (3rd experiment) SHOULDN'T IT BE ON WEDNESDAY 20th?
beads preparation
Fractions and non-fractioned extracts were adjusted with 10mM TrisHCl to 200µL volume. 5µL of green Lumafluor X beads were added to each vial and incubated @ 37°C on shaker in dark, and subsequently spun 1hr @ 14,000 xg. Supernantant removed and Matrigel added in following quantities (using injection needle to mix):
#0 - 80µL #1 - 15µL #2 - 15µL #3 - 15µL #4 - 15µL #5 - 15µL F - 15µL A - 15µL
tissue dissection
E33 ferret embryos dissected on ice, brains removed to aCSF ice-slush. Then cortex (for Joseph) and ganglionic eminences were dissected into aCSF ice-slush. Seven GE pieces were diced with scalpel blade, triturated couple of times with 1mL pipette tip and kept in aCSF on ice.
beads deposition
Landmarks made of 2µL deposits of control beads in Matrigel were placed on each glass coverslips to mark the orientation. Deposits of 5µL of each beads preparation were placed as below:
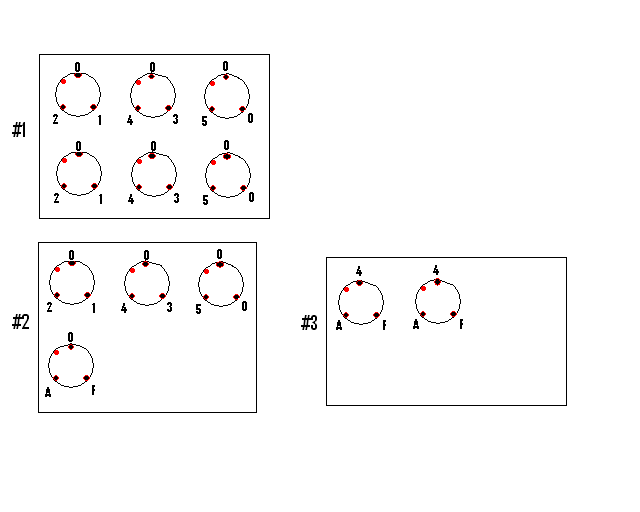
GE explants deposition
After beads deposits set (partially drying!) 4-6 explants of GE were transferred onto the center of each coverslip, equidistant from beads deposits, and after short dry-up (to reduce fluid volume) explants were covered with 5µL of fresh Matrigel.
After 10-15min in the incubator allowed for Matrigel setting, Matrigel was used to form bridges between explants and beads and then to cover entire coverslips.
After next 10-15min in the incubator NeuroBasal medium was dropped onto coverslips just enough to cover them entirely, plus ~250µL added outside glass into well.
incubation
1st 24hr (Wed-Thu)
The plate #3 was used for real-time observation and image acquisition in the Zeiss microscope incubator. The AxioVision has been set for imaging. Next day the plate was severely dried, despite extra water in between wells, and almost no sign of migration were seen in the movie. Drying was due to improper use of the internal chamber. Brian Svedberg instructed that the plate cover should have been retained on the plate under the chamber cover.
2nd 24hr (Thu-Fri)
The plate #1 was used with extra water in between wells, and with internal chamber properly assembled (drying!). Out of the incubator, it showed quite intense migration. The AxioVision has been set for imaging. Excellent migration movie next day.
3rd 24hr (Fri-Sat)
Image recording failed at 3 cycle (during nightime). I came in on Sat and fixed all three plates.