Zeina and Afrah's Research Project
NOTE: PAGE STILL UNDER CONSTRUCTION
Authors: Zeina Siam and Afrah Shafquat
Combining Aptamer Utilization and Tissue Engineering to Improve Coronary stents
We are highly interested in engineering previously designed biomaterials to optimize their functions. Our interest in cardiovascular diseases led us to choose drug eluting stents as our focus. Biodegradable stents are composed only from polymers. They have high potential to substitute for more metallic stents, since they possess reduced immunity response and can both carry and deliver much higher drug loadings. Yet, one major drawback for them is their long term thrombosis. We decided to tackle that drawback hoping to design stents that group all the efforts of various research groups and ours to process more useful stents, through the incorporation of aptamer bound matrices.
Introduction
Coronary Stents are small tubes that are inserted in the arteries to cure or prevent diseases. They may vary in composition (metals to polymers) and, (spiral to braided), and function. We aim at optimizing stents that elute plaque digesting drug.
Our stent will be elongated and braided: A paper by Isotalo et al. showed that the degradation of a braided stent is more controlled than that of a spiral one. Degradation will be our way of reducing the inflammatory effect of the stent (The stent will degrade within a year). But beyond Isotalo, we will elongate our stent, since the longer is its structure, the more capable it is of withstanding high pressure. Since the diameter varies, we can only control the length. We are also assuming that a braided one will be less susceptible to swelling, which may be one problem associated with spiral stents.
Our stent will be made from PLGA [=poly(D,L lactic-co-glycolic acid)] as suggested in (5), and our anti-plaque drug that will be released over time. Wang et al, showed in a study that PLGA has controlled release of drugs, something necessary for our anti-plaquedrugs. We will, based on a suggestion in a paper by Zuwein et al., create nanofiber matrices of these polymers to align them for enhanced pressure withstanding on the nano-level.
Approach to reduce late thrombosis : Aptamers
There has been previous attempts to make the stent release anticoagulant drugs. However, attempts were not highly successful due to several reasons such as (Balakrishnan et al):
1. Instant dilution of drug upon release and flow alterations: The speed by which drugs are diluted is highly dependent on blood stream, which fluctuates.
2. Asymmetric contents of the drug on the stent: When the drug is eluted, it is washed down by the blood, hence the concentration of the drugs tend to be higher downstream that upstream. The drug will also be asymmetrically distributed at the stent.
We here propose the approach of having the stents pick up the anticoagulants rather than releasing them through coating our stent internal walls with an aptamer matrix.
Farokhzad et al. provided a great set of reasons for using aptamers over other ligands in their referenced paper, which gave us insight to employ what they used for cancer targeting into stents: Aptamers are suitable as targeting molecules because they are nonimmunogenic, stable in a wide range of pH (≈4–9), temperature, and organic solvents. Furthermore, Aptamer synthesis does not rely on biological systems. Hence combining all these ideas, we aim at isolating aptamers that are highly specific for anticoaguant drugs (with an affinity between 30 and 80 nM).
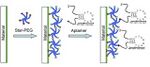
[[Image:== Coating with aptamers ==
Note : The selection of PEG star molecular was based on a paper by Avci-Adali et al. (see references for paper) Using the SELEX technology, aptamers with high affinity to anticoagulants will be generated. These aptamers will be via a hemocompatible star-PEG coating. Star-PEG contains isocyanate groups, which can react with amino groups to couple onto the material surface. Accordingly, selected aptamers will be modified at 3' -terminal with a carboxyl group so they can through peptide bonds bind to the aminogroups of star-PEG. This will be the coating of the inner surface of the stent:
1. itself will disfavor clotting, (matrix already enriched with aptamers).
2. bring ingested anticoagulants in close proximity with the stent, therefore inhibiting the accumulation of blood clots if they start forming.
How to asses whether our experiment worked
Stents will be implanted in mice and removed at regular intervals. One mouse group will not have any aptamers on its stent. One mouse group will have aptamers but will receive no anticoagulants. One more will group have aptamers and receive anticoagulants.
The first group is to investigate whether aptamers inhibit blood clotting or not. This will be assessed by tracking the amount of fibrin formation
We expect to see a decline in the clot formation on stent walls. If we see no differences between first and second group, then some hypothetical reasons may be the cause, including:
1. High pressure disrupts aptamer structure
2. High pressure causes PEG-aptamer complexes to degrade
3. Aptamers may have bound to something else in the blood, or not specific enough to bind to anticoagulant at the amounts taken
Approach: Replacement may be with higher-affinity aptamers with proteins. Proteins are harder to find or design and come with higher costs.
The effectiveness of the aptamers-anticoagulant complexed will be assessed by comparing the second group with the third group. Two possible outcomes:
1. Decrease in clotting formation. This means that our hypothesis was fulfilled
2. No difference. May be due to:
- The function of aptmer binding to anti-coagulant was not effective
- Drug amounts were not enough
We will assess amount of fibrin formed along the stent length, expectations may be:
1. Stable: the relative amount of clots formed, is any, would be stable across the length of the stent.
2. Decrease along the stent axis: Because of the asymmetric distribution of drugs where the aptamers upstream receive more drugs, since they are exposed to the drugs first, and binding to it, they decrease its apparent blood concentration making it both kinetically and thermodynamically more difficult for other aptamers to find and bind to.
If we see this, then our next step would be varying aptamer concentration per area along the stent length, inorder to maintain homeostasis.
References
1. Isolato et al. (2005): Biocompatibility properties of a new braided biodegradable urethral stent: a comparison with a biodegradable spiral and a braided metallic stent in the rabbit urethra
2. Saltzman: Building Drug delivery Into Tissue Engineering
3. MA et al. (2005): Potential of Nanofiber Matrix as Tissue-Engineering Scaffolds
4. Nugent et al. (2001): Endothelial Implants Provide Long-Term Control of Vascular Repair in a Porcine Model of Arterial Injury
5. Farokhzad, O et al (2006): Nanoparticle-Aptamer Bioconjugates: A New Approach for Targeting Prostate Cancer Cells
6. Avci-Adalia et al (2008) New strategies for in vivo tissue engineering by mimicry of homing factors for self-endothelialisation of blood contacting materials